Deck 3: Molecules in Motion: Conformations by Rotation
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/84
Play
Full screen (f)
Deck 3: Molecules in Motion: Conformations by Rotation
1
Which of the following conformations exhibits the highest torsional strain?
A)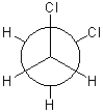
B)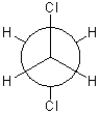
C)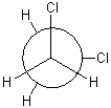
D)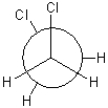
A)

B)

C)

D)


2
Which of the following Newman projections is in a gauche conformation?
A)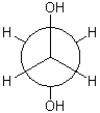
B)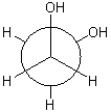
C)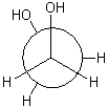
D)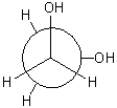
A)

B)

C)

D)


3
Which of the following represents the order of conformations in a cyclohexane ring flip?
A)chair half chair twist boat boat twist boat half chair chair
B)chair half chair boat twist boat boat half chair chair
C)chair twist boat half chair boat half chair twist boat chair
D)chair twist boat boat half chair boat twist boat chair
A)chair half chair twist boat boat twist boat half chair chair
B)chair half chair boat twist boat boat half chair chair
C)chair twist boat half chair boat half chair twist boat chair
D)chair twist boat boat half chair boat twist boat chair
chair half chair twist boat boat twist boat half chair chair
4
Which of the following conformations of cyclohexane is highest in energy?
A)chair
B)half-chair
C)boat
D)twist-boat
A)chair
B)half-chair
C)boat
D)twist-boat

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following molecules has two equally stable chair conformations?
A)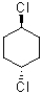
B)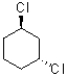
C)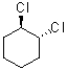
D)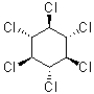
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following conformations exhibits the largest steric strain?
A)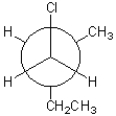
B)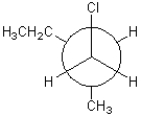
C)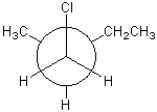
D)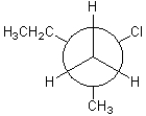
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following best describes cyclohexane in the chair conformation?
A)It contains torsional strain.
B)It contains angle strain.
C)It contains both torsional and angle strain.
D)It contains neither torsional nor angle strain.
A)It contains torsional strain.
B)It contains angle strain.
C)It contains both torsional and angle strain.
D)It contains neither torsional nor angle strain.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following best describes the bromo group in the molecule shown below? 
A)It is equatorial and above the plane of the ring.
B)It is equatorial and below the plane of the ring.
C)It is axial and above the plane of the ring.
D)It is axial and below the plane of the ring.

A)It is equatorial and above the plane of the ring.
B)It is equatorial and below the plane of the ring.
C)It is axial and above the plane of the ring.
D)It is axial and below the plane of the ring.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following molecules exhibits the least ring strain?
A)cyclobutane
B)cyclopentane
C)cyclohexane
D)cycloheptane
A)cyclobutane
B)cyclopentane
C)cyclohexane
D)cycloheptane

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
10
What is the relationship between the bromo and chloro substituents in the molecule shown below? 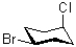
A)cis
B)trans
C)syn
D)anti

A)cis
B)trans
C)syn
D)anti

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is a chair conformation of the structure shown below? 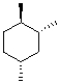
A)
B)
C)
D)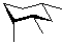

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following molecules has two equally stable chair conformations?
A)trans-1,4-dimethylcyclohexane
B)cis-1,4-dimethylcyclohexane
C)trans-1-ethyl-4-methylcyclohexane
D)cis-1-ethyl-4-methylcyclohexane
A)trans-1,4-dimethylcyclohexane
B)cis-1,4-dimethylcyclohexane
C)trans-1-ethyl-4-methylcyclohexane
D)cis-1-ethyl-4-methylcyclohexane

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following best describes a cyclopropane ring?
A)It contains torsional strain.
B)It contains angle strain.
C)It contains both torsional and angle strain.
D)It contains neither torsional nor angle strain.
A)It contains torsional strain.
B)It contains angle strain.
C)It contains both torsional and angle strain.
D)It contains neither torsional nor angle strain.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following best describes the molecule shown below? 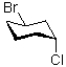
A)Both bromo and chloro are axial.
B)Both bromo and chloro are equatorial.
C)Bromo is axial and chloro is equatorial.
D)Bromo is equatorial and chloro is axial.

A)Both bromo and chloro are axial.
B)Both bromo and chloro are equatorial.
C)Bromo is axial and chloro is equatorial.
D)Bromo is equatorial and chloro is axial.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following represents a Newman projection of the molecule shown below? 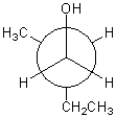
A)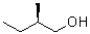
B)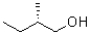
C)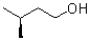
D)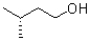

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following represents the Newman projection shown below? 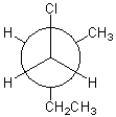
A)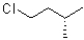
B)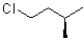
C)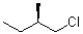
D)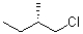

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following exhibits the largest angle strain?
A)cyclobutane
B)cyclopentane
C)cyclohexane
D)cycloheptane
A)cyclobutane
B)cyclopentane
C)cyclohexane
D)cycloheptane

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
18
What is the optimal dihedral angle between substituents of a Newman projection in a staggered conformation?
A)15°
B)30°
C)60°
D)90°
A)15°
B)30°
C)60°
D)90°

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following represents the most stable chair conformation of the molecule shown below? 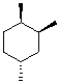
A)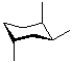
B)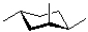
C)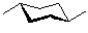
D)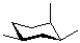

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following represents the most stable conformation of cis-1-bromo-2-chlorocyclohexane?
A)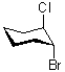
B)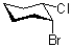
C)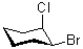
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is a Newman conformation of the structure shown below? 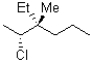
A)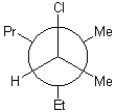
B)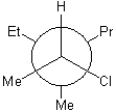
C)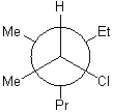
D)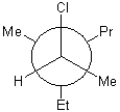

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
22
How many possible butterfly conformations exist for cyclobutane?
A)1
B)2
C)4
D)8
A)1
B)2
C)4
D)8

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following best describes the Newman conformation shown below? 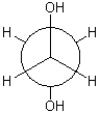
A)gauche
B)eclipsed
C)antiperiplanar
D)torsional

A)gauche
B)eclipsed
C)antiperiplanar
D)torsional

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
24
What is the torsional angle between the alcohol and methyl substituents shown below? 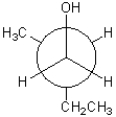
A)30°
B)60°
C)90°
D)120°

A)30°
B)60°
C)90°
D)120°

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is a Newman projection of the molecule shown below? 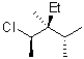
A)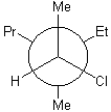
B)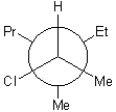
C)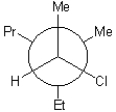
D)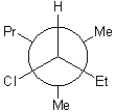

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following does NOT represents a ring flip of the chair structure shown below? 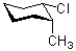
A)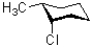
B)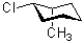
C)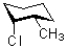
D)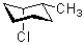

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following best describes the conformation of a cyclobutane ring?
A)envelope
B)chair
C)boat
D)butterfly
A)envelope
B)chair
C)boat
D)butterfly

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is a Newman projection of the molecule shown below? 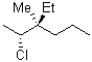
A)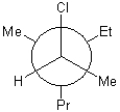
B)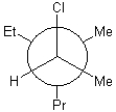
C)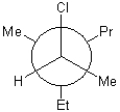
D)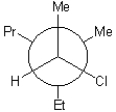

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
29
How many possible envelope conformations exist for cyclopentane?
A)1
B)2
C)5
D)10
A)1
B)2
C)5
D)10

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following best describes the conformation of a cyclopentane ring?
A)envelope
B)chair
C)boat
D)butterfly
A)envelope
B)chair
C)boat
D)butterfly

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
31
Which group would you expect to have the largest A-value?
A)methyl
B)ethyl
C)isopropyl
D)tert-butyl
A)methyl
B)ethyl
C)isopropyl
D)tert-butyl

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following represents a conformation of the molecule shown below? 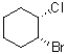
A)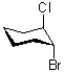
B)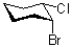
C)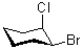
D)

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following best describes the relationship between bromo and chloro in the molecule shown below? 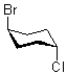
A)cis
B)trans
C)syn
D)anti

A)cis
B)trans
C)syn
D)anti

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following represents a ring flip of the chair structure shown below? 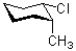
A)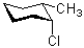
B)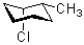
C)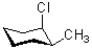
D)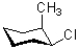

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following has the most conformational stability?
A)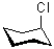
B)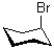
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following best describes the molecule shown below? 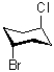
A)Both bromo and chloro are axial.
B)Both bromo and chloro are equatorial.
C)Bromo is axial and chloro is equatorial.
D)Bromo is equatorial and chloro is axial.

A)Both bromo and chloro are axial.
B)Both bromo and chloro are equatorial.
C)Bromo is axial and chloro is equatorial.
D)Bromo is equatorial and chloro is axial.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
37
What best describes the relationship between bromo and chloro in the molecule shown below? 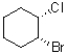
A)cis
B)trans
C)syn
D)anti

A)cis
B)trans
C)syn
D)anti

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following molecules contains the most rotatable bonds?
A)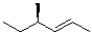
B)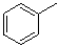
C)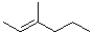
D)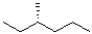
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
39
What is the torsional angle between the chloro and propyl substituents shown below? 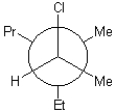
A)15°
B)30°
C)45°
D)60°

A)15°
B)30°
C)45°
D)60°

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
40
What is the interior angle of a cyclobutane ring in its most stable conformation?
A)less than 90°
B)90°
C)greater than 90°
D)impossible to determine
A)less than 90°
B)90°
C)greater than 90°
D)impossible to determine

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
41
A cyclohexane twist-boat conformation is more strained then the half-chair conformation.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
42
A Newman projection with eclipsed substituents is considered gauche.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
43
The chair conformations of trans-1,4-dimethylcyclohexane are equal in energy.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
44
Free rotation is allowed around single bonds.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
45
Cyclobutane rings adopt an envelope conformation.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
46
The chair conformation is the most stable conformation of cyclohexane rings.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
47
An eclipsed confirmation has only steric strain.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
48
Large substituents prefer to point equatorial in cyclohexane chair conformations.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
49
The most stable conformation of cyclopropane is planar.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
50
The chair conformations of trans-1,3-dimethylcyclohexane are equal in energy.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
51
Axial substituents exist above and below the plane of the ring.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
52
The interpenetration of electron clouds from nearby substituents is a result of torsional strain.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
53
A cyclohexane twist-boat conformation is more strained than the boat conformation.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
54
A cyclohexane boat conformation is more strained than the half chair conformation.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
55
Which cyclopropane ring would be more strained and why? 
A)A because of increased angle strain
B)A because of increased torsional strain
C)B because of increased angle strain
D)B because of increased torsional strain

A)A because of increased angle strain
B)A because of increased torsional strain
C)B because of increased angle strain
D)B because of increased torsional strain

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
56
Cyclopentane rings have 10 possible envelope conformations available to them.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
57
Cyclopentane rings adopt a boat conformation.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
58
The most stable conformation of cyclobutane is planar.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
59
Ring-flips of cyclohexane ring chair conformations result in an inversion of stereochemistry.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
60
The chair conformations of cis-1,3-dimethylcyclohexane are equal in energy.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
61
Cyclobutane adopts a_______________ conformation.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
62
Cyclohexane can undergo chair flips rapidly at room temperature.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
63
The difference in energy between two chair conformations is referred to as an _______________.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
64
A gauche confirmation leads to _______________ strain.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
65
Cyclopropane has a lower heat of combustion then propane.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
66
Draw the Newman projection of the following molecule looking through the bond indicated by a *. 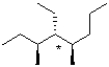


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
67
The interpenetration of electron clouds from nearby substituents is a result of_______________ strain.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
68
The _______________ is the highest energy cyclohexane conformation.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
69
Cyclopentane rings adopt a _______________ conformation.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
70
Chair conformations still experience angle strain.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
71
The boat conformation is the least stable cyclohexane conformation.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
72
A Newman projection whereupon the dihedral angle between two substituents is 180° is said to be in a _______________ conformation.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
73
Draw both chair conformations of the molecule shown below and indicate which is more stable. 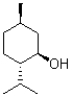


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
74
Draw the Newman projection of the following molecule looking through the bond indicated by a *. 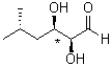


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
75
A Newman projection whereupon the dihedral angle between two substituents is 0° is said to be in a _______________ conformation.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
76
Draw the ring flip of the following molecule,shown below. 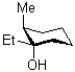


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
77
Molecules that exist in different conformations are called _______________ .

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
78
Axial substituents in a chair conformation experience steric strain through 1,2-diaxial interactions

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
79
A-values represent the torsional strain in chair conformations.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
80
A Newman projection whereupon the dihedral angle between two substituents is 180° is said to be in a _______________ conformation.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck



