Deck 4: Stereochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/83
Play
Full screen (f)
Deck 4: Stereochemistry
1
What best describes the relationship between I and II shown below? 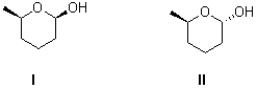
A)identical molecules
B)enantiomers
C)diastereomers
D)constitutional isomers
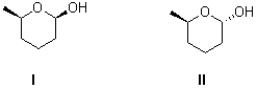
A)identical molecules
B)enantiomers
C)diastereomers
D)constitutional isomers
diastereomers
2
What best describes the molecule shown below? 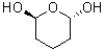
A)symmetrical and chiral
B)non-symmetrical and chiral
C)symmetrical and achiral
D)non-symmetrical and achiral

A)symmetrical and chiral
B)non-symmetrical and chiral
C)symmetrical and achiral
D)non-symmetrical and achiral
non-symmetrical and chiral
3
Which of the following describes the three molecules shown below? 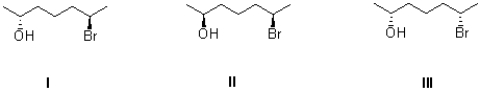
A)I and II are diastereomers.
B)I and III are enantiomers.
C)II and III are diastereomers.
D)I,II,and III are all diastereomers with each other.

A)I and II are diastereomers.
B)I and III are enantiomers.
C)II and III are diastereomers.
D)I,II,and III are all diastereomers with each other.
I and II are diastereomers.
4
How many chiral centres are there in 7-dehydrocholesterol (shown below)? 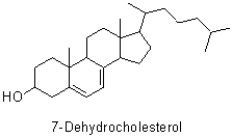
A)0
B)3
C)7
D)9

A)0
B)3
C)7
D)9

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
5
How many stereoisomers does the following molecule contain? 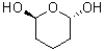
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following (I,II,III)is an enantiomer of the molecule shown below? 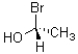
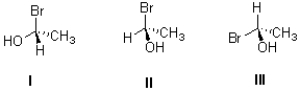
A)only I
B)only II
C)both II and III
D)both I and II


A)only I
B)only II
C)both II and III
D)both I and II

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
7
What best describes the absolute configuration of the epoxide shown below. 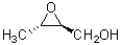
A)2R,3R
B)2R,3S
C)2S,3R
D)2S,3S

A)2R,3R
B)2R,3S
C)2S,3R
D)2S,3S

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
8
What best describes the relationship between I and II shown below? 
A)identical molecules
B)enantiomers
C)diastereomers
D)constitutional isomers

A)identical molecules
B)enantiomers
C)diastereomers
D)constitutional isomers

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
9
What best describes the relationship between I and II shown below? 
A)identical molecules
B)enantiomers
C)diastereomers
D)constitutional isomers

A)identical molecules
B)enantiomers
C)diastereomers
D)constitutional isomers

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
10
What term refers to two molecules that differ at some but not all chiral centres?
A)enantiomers
B)diastereomers
C)constitutional isomers
D)atropisomers
A)enantiomers
B)diastereomers
C)constitutional isomers
D)atropisomers

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
11
What best describes the molecule shown below? 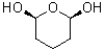
A)symmetrical and chiral
B)non-symmetrical and chiral
C)symmetrical and achiral
D)non-symmetrical and achiral

A)symmetrical and chiral
B)non-symmetrical and chiral
C)symmetrical and achiral
D)non-symmetrical and achiral

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following describes the three molecules shown below? 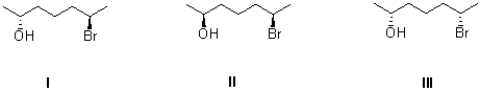
A)I and II are enantiomers.
B)I and III are enantiomers.
C)II and III are enantiomers.
D)I,II,and III are not enantiomers with each other.

A)I and II are enantiomers.
B)I and III are enantiomers.
C)II and III are enantiomers.
D)I,II,and III are not enantiomers with each other.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following hexoses are distereomers with each other? 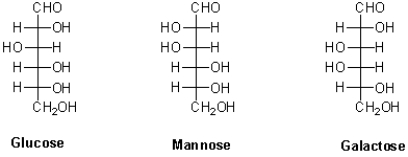
A)Glucose and mannose are diastereomers.
B)Glucose and galactose are diastereomers.
C)Galactose and mannose are diastereomers.
D)Glucose,mannose,and galactose are diastereomers with each other.

A)Glucose and mannose are diastereomers.
B)Glucose and galactose are diastereomers.
C)Galactose and mannose are diastereomers.
D)Glucose,mannose,and galactose are diastereomers with each other.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
14
What is the correct IUPAC name for the following compound? 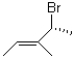
A)(R,E)-4-bromo-3-methylpent-2-ene
B)(S,E)-4-bromo-3-methylpent-2-ene
C)(R,Z)-4-bromo-3-methylpent-2-ene
D)(S,Z)-4-bromo-3-methylpent-2-ene

A)(R,E)-4-bromo-3-methylpent-2-ene
B)(S,E)-4-bromo-3-methylpent-2-ene
C)(R,Z)-4-bromo-3-methylpent-2-ene
D)(S,Z)-4-bromo-3-methylpent-2-ene

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
15
What best describes the relationship between I and II shown below? 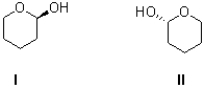
A)identical molecules
B)enantiomers
C)diastereomers
D)constitutional isomers
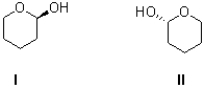
A)identical molecules
B)enantiomers
C)diastereomers
D)constitutional isomers

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
16
What is the absolute configuration of the molecule shown below? 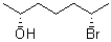
A)2S,6S
B)2S,6R
C)2R,6S
D)2R,6R

A)2S,6S
B)2S,6R
C)2R,6S
D)2R,6R

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
17
What can be said about L-Glyceraldehyde (shown below)? 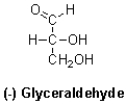
A)It rotates plane-polarized light clockwise and has an absolute configuration of R.
B)It rotates plane-polarized light counter-clockwise and has an absolute configuration of R.
C)It rotates plane-polarized light clockwise and has an absolute configuration of S.
D)It rotates plane-polarized light counter-clockwise and has an absolute configuration of S.

A)It rotates plane-polarized light clockwise and has an absolute configuration of R.
B)It rotates plane-polarized light counter-clockwise and has an absolute configuration of R.
C)It rotates plane-polarized light clockwise and has an absolute configuration of S.
D)It rotates plane-polarized light counter-clockwise and has an absolute configuration of S.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
18
What is the absolute configuration around the chiral centre of the molecule shown below? 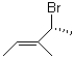
A)Choice R
B)Choice S
C)Choice E
D)Choice Z

A)Choice R
B)Choice S
C)Choice E
D)Choice Z

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following (I,II,III)is the Fischer projection of the compound shown below? 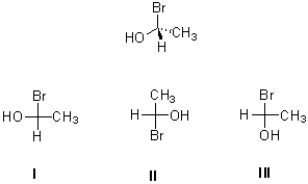
A)only I
B)only II
C)both II and III
D)both I and III

A)only I
B)only II
C)both II and III
D)both I and III

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following best describes the stereochemical relationship between the hexoses shown below? 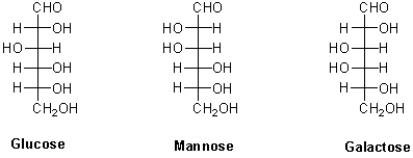
A)Glucose and mannose are enantiomers.
B)Glucose and galactose are enantiomers.
C)Galactose and mannose are enantiomers.
D)Glucose and mannose are diastereomers.

A)Glucose and mannose are enantiomers.
B)Glucose and galactose are enantiomers.
C)Galactose and mannose are enantiomers.
D)Glucose and mannose are diastereomers.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
21
What property to enantiomers differ by?
A)boiling point
B)optical rotation
C)polarity
D)solubility
A)boiling point
B)optical rotation
C)polarity
D)solubility

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
22
Figure 1
Figure 1: Epoxides I,II,III,IV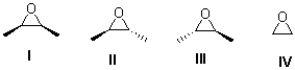
Which of the following compounds in Figure 1 is/are meso?
A)I
B)IV
C)I and IV
D)II and III
Figure 1: Epoxides I,II,III,IV

Which of the following compounds in Figure 1 is/are meso?
A)I
B)IV
C)I and IV
D)II and III

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
23
Figure 1
Figure 1: Epoxides I,II,III,IV
Which of the following compounds in Figure 1 contains no chiral centres?
A)I
B)II and III
C)IV
D)I and IV
Figure 1: Epoxides I,II,III,IV

Which of the following compounds in Figure 1 contains no chiral centres?
A)I
B)II and III
C)IV
D)I and IV

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
24
What are the absolute configurations of the chiral centres in the molecule shown below? 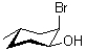
A)1S,2R,4R
B)1S,2R,4S
C)1R,2S,4R
D)1R,2R,4S

A)1S,2R,4R
B)1S,2R,4S
C)1R,2S,4R
D)1R,2R,4S

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following best describes the stereochemical relationship of the structures shown below? 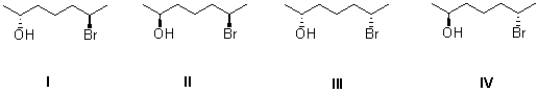
A)I and II are enantiomers,and III and IV are diastereomers.
B)I and IV are enantiomers,and II and III are diastereomers.
C)II and III are enantiomers,and I and IV are diastereomers.
D)I and IV are enantiomers,and II and III are meso.

A)I and II are enantiomers,and III and IV are diastereomers.
B)I and IV are enantiomers,and II and III are diastereomers.
C)II and III are enantiomers,and I and IV are diastereomers.
D)I and IV are enantiomers,and II and III are meso.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
26
What is the relationship between the stereoisomers I,II,and III? 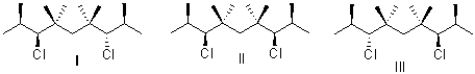
A)I is a meso compound;II and III are diastereomers.
B)II is a meso compound;I and III are enantiomers.
C)II is a meso compound;I and III are diastereomers.
D)III is a meso compound;I and II are enantiomers.

A)I is a meso compound;II and III are diastereomers.
B)II is a meso compound;I and III are enantiomers.
C)II is a meso compound;I and III are diastereomers.
D)III is a meso compound;I and II are enantiomers.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
27
How many stereoisomers does the following compound have? 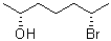
A)2
B)3
C)4
D)8

A)2
B)3
C)4
D)8

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
28
Figure 1
Figure 1: Epoxides I,II,III,IV
Which of the following epoxides in Figure 1 is/are achiral?
A)IV
B)II,III
C)I,IV
D)II,III,IV
Figure 1: Epoxides I,II,III,IV

Which of the following epoxides in Figure 1 is/are achiral?
A)IV
B)II,III
C)I,IV
D)II,III,IV

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
29
What are the absolute configurations of the chiral centres in the molecule shown below? 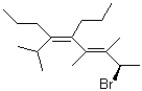
A)2R,3E,5Z
B)2R,3Z,5E
C)2S,3E,5Z
D)2S,3Z,5E

A)2R,3E,5Z
B)2R,3Z,5E
C)2S,3E,5Z
D)2S,3Z,5E

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following compounds is optically active? 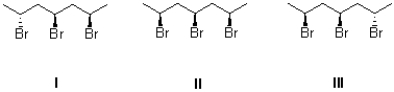
A)I and III
B)II
C)II and III
D)I,II,and III

A)I and III
B)II
C)II and III
D)I,II,and III

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
31
What is the relationship between the two molecules shown below? 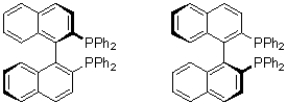
A)identical molecules
B)enantiomers
C)diastereomers
D)atropospmers

A)identical molecules
B)enantiomers
C)diastereomers
D)atropospmers

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
32
Figure 1
Figure 1: Epoxides I,II,III,IV
Which of the following pairs in Figure 1 are enantiomers?
A)III and IV
B)II and III
C)I and II
D)I and IV
Figure 1: Epoxides I,II,III,IV

Which of the following pairs in Figure 1 are enantiomers?
A)III and IV
B)II and III
C)I and II
D)I and IV

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
33
Figure 1
Figure 1: Epoxides I,II,III,IV
In Figure 1,what is the absolute configuration of epoxide II?
A)2R 3R
B)2R 3S
C)2S 3R
D)2S 3S
Figure 1: Epoxides I,II,III,IV

In Figure 1,what is the absolute configuration of epoxide II?
A)2R 3R
B)2R 3S
C)2S 3R
D)2S 3S

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following compounds is considered meso? 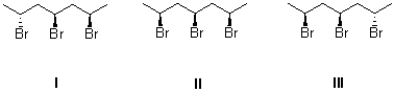
A)I
B)II
C)III
D)I and III

A)I
B)II
C)III
D)I and III

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following statements best describes the molecule shown below? 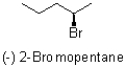
A)It rotates plane-polarized light clockwise and has an absolute configuration of R.
B)It rotates plane-polarized light counter-clockwise and has an absolute configuration of R.
C)It rotates plane-polarized light clockwise and has an absolute configuration of S.
D)It rotates plane polarized light counter-clockwise and has an absolute configuration of S.

A)It rotates plane-polarized light clockwise and has an absolute configuration of R.
B)It rotates plane-polarized light counter-clockwise and has an absolute configuration of R.
C)It rotates plane-polarized light clockwise and has an absolute configuration of S.
D)It rotates plane polarized light counter-clockwise and has an absolute configuration of S.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following molecules is achiral?
A)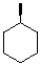
B)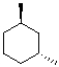
C)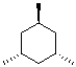
D)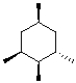
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
37
Figure 1
Figure 1: Epoxides I,II,III,IV
Which of the following epoxides in Figure 1 is/are optically active?
A)I
B)II and III
C)I,II,and III
D)I and IV
Figure 1: Epoxides I,II,III,IV

Which of the following epoxides in Figure 1 is/are optically active?
A)I
B)II and III
C)I,II,and III
D)I and IV

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
38
What is the ratio of stereoisomers in a mixture that contains an enantiomeric excess of 20% R stereoisomer isomer?
A)20% R,80% S
B)40% R,60% S
C)60% R,40% S
D)80% R,20% S
A)20% R,80% S
B)40% R,60% S
C)60% R,40% S
D)80% R,20% S

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
39
If a sample contains 70% of the (+)isomer and 30% of the (-)isomer,what is its enantiomeric excess?
A)70% (+)
B)40% (+)
C)30% (+)
D)30% (-)
A)70% (+)
B)40% (+)
C)30% (+)
D)30% (-)

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
40
Figure 1
Figure 1: Epoxides I,II,III,IV
In Figure 1,what is the absolute configuration of epoxide III?
A)2R 3R
B)2R 3S
C)2S 3R
D)2S 3S
Figure 1: Epoxides I,II,III,IV

In Figure 1,what is the absolute configuration of epoxide III?
A)2R 3R
B)2R 3S
C)2S 3R
D)2S 3S

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
41
Optical purity is used to determine enantiomeric excess.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
42
Only carbon can be a chiral centre.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
43
Z stereochemistry represents the highest-priority groups being on the same side of an alkene.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
44
Meso compounds contain an internal plane of symmetry.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
45
Any molecule that has a non-superimposable mirror image is considered chiral.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
46
According to the Cahn Ingold Prelog rules,isotopes are not considered when assigning substituent priority.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
47
Two molecules that differ at some but not all chiral centres are referred to as diastereomers.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
48
If the penultimate carbon of a carbohydrate has the hydroxyl group pointing right,it is considered a D sugar.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
49
2-bromo-3-methylbutane has four stereoisomers.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
50
Diastereomers have identical physical properties.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
51
Molecules that rotate plane-polarized light counter-clockwise are denoted as L.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
52
Any compound that has a chiral centre is considered a chiral molecule.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
53
R absolute configuration represents a counter-clockwise arrangement of the highest-priority substituents.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
54
In a Fischer projection,the horizontal bonds are assumed to go into the page.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
55
Molecules that rotate plane-polarized light clockwise have an absolute configuration of R.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
56
A molecule that contains an internal mirror plane is considered meso and achiral.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
57
A molecule is chiral if it possesses a superimposable mirror image.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
58
According to the Cahn-Ingold-Prelog rules,lone pairs electrons have a lower priority than a hydrogen atom.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
59
2-bromo-3-methylbutane has two chiral centres.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
60
Specific rotation takes the concentration of a sample into account.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
61
E and Z are types of stereochemistry.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
62
Determine the stereochemical relationship of II with each of the other seven molecules.(Enantiomers,Diastereomers,Identical molecules) 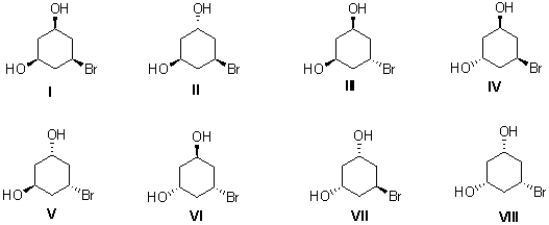


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
63
Identify the absolute configuration of the chiral centres in the following compound. 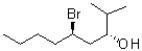


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
64
A 50:50 sample of enantiomers is also known as a _______________ mixture.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
65
A molecule with chiral centres and an internal plane of symmetry is considered _______________.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
66
Bonds that go into the page are represented in the _______________ direction on a Fischer projection.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
67
A _______________ is when molecules are drawn in two dimensions in a cross-like format

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
68
If the highest-priority substituents are on opposite sides of an alkene,it is designated with the Z configuration.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
69
Draw glucose in the zig-zag template shown below,given the Fischer projection. 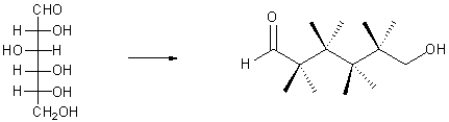


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
70
Enantiomers have _______________ physical properties.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
71
Stereoisomers that are a result of hindered rotation are referred to as _______________ .

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
72
Stereoisomers that differ at some but not all chiral centres are referred to as _______________.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
73
A racemic mixture of 2-butanol can have a different boiling point than its individual enantiomers.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
74
The maximum number of stereoisomers a compound can have is _______________ where n is the number of chiral centres.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
75
Determine the absolute configuration of the chiral centres in the molecule shown below. 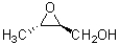


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
76
Stereoisomers are a type of constitutional isomer.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
77
Stereoisomers that differ at all chiral centres are referred to as _______________ .

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
78
A molecule name preceded by (+)rotates plane-polarized light _______________ .

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
79
Meso compounds increase the number of possible stereoisomers.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
80
Convert the chair structure I into a line structure,and determine its stereochemical relationship to II shown below. 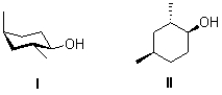


Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck



