Deck 1: Carbon and Its Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/84
Play
Full screen (f)
Deck 1: Carbon and Its Compounds
1
What best represents the C-C bond in C2H6?
A)s-sp3 orbital overlap
B)sp3-sp3 orbital overlap
C)s-s orbital overlap
D)p-p orbital overlap
A)s-sp3 orbital overlap
B)sp3-sp3 orbital overlap
C)s-s orbital overlap
D)p-p orbital overlap
sp3-sp3 orbital overlap
2
Figure 2
The following questions refer to the structure of heroin (shown below).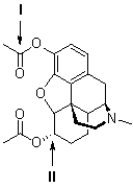
Referring to Figure 2,what is the hybridization of the carbon atom at II?
A)p
B)sp
C)sp2
D)sp3
The following questions refer to the structure of heroin (shown below).

Referring to Figure 2,what is the hybridization of the carbon atom at II?
A)p
B)sp
C)sp2
D)sp3
sp3
3
Figure 2
The following questions refer to the structure of heroin (shown below).
Referring to Figure 2,what is the geometry of the carbon atom shown at II?
A)bent
B)trigonal planar
C)tetrahedral
D)trigonal pyramidal
The following questions refer to the structure of heroin (shown below).

Referring to Figure 2,what is the geometry of the carbon atom shown at II?
A)bent
B)trigonal planar
C)tetrahedral
D)trigonal pyramidal
tetrahedral
4
Figure 1 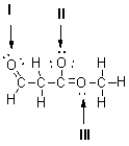
Referring to Figure 1,what is the formal charge of the oxygen atom at I?
A)+1
B)0
C)-1
D)-2

Referring to Figure 1,what is the formal charge of the oxygen atom at I?
A)+1
B)0
C)-1
D)-2

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
5
Which element or ion has the following electron configuration: 1s22s22p63s23s4?
A)S
B)O
C)Ar
D)Si
A)S
B)O
C)Ar
D)Si

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
6
How many bonded and non-bonded electrons does a molecule with no formal charges and the formula C2H4O2 contain?
A)4 bonded,4 non-bonded
B)7 bonded.4 non-bonded
C)7 bonded,5 non-bonded
D)4 bonded,7 non-bonded
A)4 bonded,4 non-bonded
B)7 bonded.4 non-bonded
C)7 bonded,5 non-bonded
D)4 bonded,7 non-bonded

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
7
How many valence electrons does a nitrogen atom contain?
A)2
B)3
C)5
D)7
A)2
B)3
C)5
D)7

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
8
How many orientations exist for a p orbital?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
9
Figure 1 
Referring to Figure 1,what is the formal charge of the oxygen atom at II?
A)+1
B)0
C)-1
D)-2

Referring to Figure 1,what is the formal charge of the oxygen atom at II?
A)+1
B)0
C)-1
D)-2

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
10
Figure 2
The following questions refer to the structure of heroin (shown below).
Referring to Figure 2,what is the geometry of the carbon atom shown at I?
A)bent
B)trigonal planar
C)tetrahedral
D)trigonal pyramidal
The following questions refer to the structure of heroin (shown below).

Referring to Figure 2,what is the geometry of the carbon atom shown at I?
A)bent
B)trigonal planar
C)tetrahedral
D)trigonal pyramidal

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
11
How many valence electrons does an O2- ion contain?
A)2
B)6
C)8
D)10
A)2
B)6
C)8
D)10

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following represents the ground state electron configuration for a O2- ion?
A)1s22s22p4
B)1s22s22p2
C)1s22s22p6
D)2s22p4
A)1s22s22p4
B)1s22s22p2
C)1s22s22p6
D)2s22p4

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
13
Figure 2
The following questions refer to the structure of heroin (shown below).
Referring to Figure 2,what is the hybridization of the nitrogen atom?
A)p
B)sp
C)sp2
D)sp3
The following questions refer to the structure of heroin (shown below).

Referring to Figure 2,what is the hybridization of the nitrogen atom?
A)p
B)sp
C)sp2
D)sp3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
14
What is the Lewis structure of a compound that has the formula of CCl3 and contains 24 valence electrons?
A)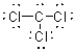
B)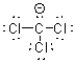
C)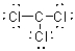
D)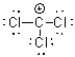
A)
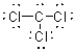
B)
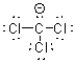
C)
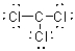
D)


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
15
How many orientations exist for a s orbital?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
16
How many orientations exist for an sp orbital?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
17
Figure 1 
Referring to Figure 1,what is the formal charge of the oxygen atom at III?
A)+1
B)0
C)-1
D)-2

Referring to Figure 1,what is the formal charge of the oxygen atom at III?
A)+1
B)0
C)-1
D)-2

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
18
Figure 2
The following questions refer to the structure of heroin (shown below).
Referring to Figure 2,what is the hybridization of the carbon atom at I?
A)p
B)sp
C)sp2
D)sp3
The following questions refer to the structure of heroin (shown below).

Referring to Figure 2,what is the hybridization of the carbon atom at I?
A)p
B)sp
C)sp2
D)sp3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
19
What best represents a C-H bond in CH4?
A)s-sp3 orbital overlap
B)sp3-sp3 orbital overlap
C)s-s orbital overlap
D)p-p orbital overlap
A)s-sp3 orbital overlap
B)sp3-sp3 orbital overlap
C)s-s orbital overlap
D)p-p orbital overlap

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
20
How many orientations exist for a sp3 orbital?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
21
Figure 3
The following questions refer to the molecule drawn below.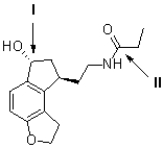
Referring to Figure 3,what is the orbital geometry of the carbon atom at I?
A)trigonal planar
B)trigonal pyramidal
C)tetrahedral
D)trigonal bipyramidal
The following questions refer to the molecule drawn below.

Referring to Figure 3,what is the orbital geometry of the carbon atom at I?
A)trigonal planar
B)trigonal pyramidal
C)tetrahedral
D)trigonal bipyramidal

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following best describes a ð orbital?
A)It is a bonding orbital with zero nodes.
B)It is an anti-bonding orbital with zero nodes.
C)It is a bonding orbital with one node.
D)It is an anti-bonding orbital with one node.
A)It is a bonding orbital with zero nodes.
B)It is an anti-bonding orbital with zero nodes.
C)It is a bonding orbital with one node.
D)It is an anti-bonding orbital with one node.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
23
How many hydrogens does the following line structure contain? 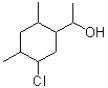
A)1
B)10
C)19
D)26

A)1
B)10
C)19
D)26

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the labelled carbons in the molecule shown below is the most electron rich? 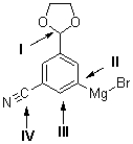
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
25
Figure 3
The following questions refer to the molecule drawn below.
Referring to Figure 3,how many sp2 atoms are contained in the molecule shown?
A)6
B)7
C)8
D)9
The following questions refer to the molecule drawn below.

Referring to Figure 3,how many sp2 atoms are contained in the molecule shown?
A)6
B)7
C)8
D)9

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
26
What best describes a wedged bond?
A)It looks like and represents going into the page.
and represents going into the page.
B)It looks like and represents going out of the page.
and represents going out of the page.
C)It looks like and represents going into the page.
and represents going into the page.
D)It looks like
A)It looks like
 and represents going into the page.
and represents going into the page.B)It looks like
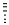 and represents going out of the page.
and represents going out of the page.C)It looks like
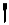 and represents going into the page.
and represents going into the page.D)It looks like

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
27
What best represents the hydroxyl group in the molecule shown below? 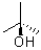
A)It is sp hybridized and pointed out of the page.
B)It is sp hybridized and pointed into the page.
C)It is sp3 hybridized and pointed out of the page.
D)It is sp3 hybridized and pointed into the page.

A)It is sp hybridized and pointed out of the page.
B)It is sp hybridized and pointed into the page.
C)It is sp3 hybridized and pointed out of the page.
D)It is sp3 hybridized and pointed into the page.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
28
How many bonds does oxygen make while remaining neutral?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
29
Figure 3
The following questions refer to the molecule drawn below.
Referring to Figure 3,how many sp3 atoms are contained in the molecule shown?
A)9
B)10
C)11
D)12
The following questions refer to the molecule drawn below.

Referring to Figure 3,how many sp3 atoms are contained in the molecule shown?
A)9
B)10
C)11
D)12

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
30
What can be said about the carbon atom at I? 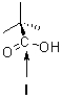
A)It is sp2 hybridized and pointed out of the page.
B)It is sp2 hybridized and pointed into the page.
C)It is sp3 hybridized and pointed out of the page.
D)It is sp3 hybridized and pointed into the page.

A)It is sp2 hybridized and pointed out of the page.
B)It is sp2 hybridized and pointed into the page.
C)It is sp3 hybridized and pointed out of the page.
D)It is sp3 hybridized and pointed into the page.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
31
Figure 3
The following questions refer to the molecule drawn below.
Referring to Figure 3,how many hydrogen atoms are contained in the molecule shown?
A)16
B)19
C)21
D)26
The following questions refer to the molecule drawn below.

Referring to Figure 3,how many hydrogen atoms are contained in the molecule shown?
A)16
B)19
C)21
D)26

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
32
Figure 3
The following questions refer to the molecule drawn below.
Referring to Figure 3,what is the orbital geometry of the carbon atom at II?
A)trigonal planar
B)trigonal pyramidal
C)tetrahedral
D)trigonal bipyramidal
The following questions refer to the molecule drawn below.

Referring to Figure 3,what is the orbital geometry of the carbon atom at II?
A)trigonal planar
B)trigonal pyramidal
C)tetrahedral
D)trigonal bipyramidal

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
33
What are the orbital angles around an sp2 hybridized atom?
A)180º
B)120º
C)109.5º
D)90º
A)180º
B)120º
C)109.5º
D)90º

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following molecules is represented in condensed structure?
A)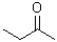
B)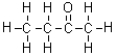
C)CH3CH2COCH3
D)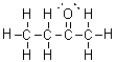
A)

B)
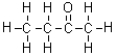
C)CH3CH2COCH3
D)
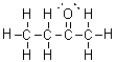

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
35
What is the geometry around an sp hybridized carbon?
A)linear
B)trigonal planar
C)tetrahedral
D)trigonal pyramidal
A)linear
B)trigonal planar
C)tetrahedral
D)trigonal pyramidal

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
36
Figure 3
The following questions refer to the molecule drawn below.
Referring to Figure 3,what is the hybridization of the carbon atom at II?
A)s
B)sp
C)sp2
D)sp3
The following questions refer to the molecule drawn below.

Referring to Figure 3,what is the hybridization of the carbon atom at II?
A)s
B)sp
C)sp2
D)sp3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
37
Figure 3
The following questions refer to the molecule drawn below.
Referring to Figure 3,what is the hybridization of the carbon atom at I?
A)s
B)sp
C)sp2
D)sp3
The following questions refer to the molecule drawn below.

Referring to Figure 3,what is the hybridization of the carbon atom at I?
A)s
B)sp
C)sp2
D)sp3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following best describes a ó* orbital?
A)It is a bonding orbital with zero nodes.
B)It is an anti-bonding orbital with zero nodes.
C)It is a bonding orbital with one node.
D)It is an anti-bonding orbital with one node.
A)It is a bonding orbital with zero nodes.
B)It is an anti-bonding orbital with zero nodes.
C)It is a bonding orbital with one node.
D)It is an anti-bonding orbital with one node.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
39
Figure 3
The following questions refer to the molecule drawn below.
Referring to Figure 3,what best represents the hydroxyl group?
A)It is sp hybridized and pointed out of the page.
B)It is sp hybridized and pointed into the page.
C)It is sp3 hybridized and pointed out of the page.
D)It is sp3 hybridized and pointed into the page.
The following questions refer to the molecule drawn below.

Referring to Figure 3,what best represents the hydroxyl group?
A)It is sp hybridized and pointed out of the page.
B)It is sp hybridized and pointed into the page.
C)It is sp3 hybridized and pointed out of the page.
D)It is sp3 hybridized and pointed into the page.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
40
What is the geometry around an sp2 hybridized carbon?
A)linear
B)trigonal planar
C)tetrahedral
D)trigonal pyramidal
A)linear
B)trigonal planar
C)tetrahedral
D)trigonal pyramidal

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
41
Electrons in ó bonds can be delocalized.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the labelled carbons in the molecule shown below is the most electron deficient? 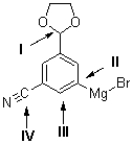
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
43
A carbanion contains a carbon atom with a formal negative charge.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
44
Carbocations break the octet rule.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
45
In a O-H bond,the electron density is skewed towards the hydrogen atom.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
46
sp3 hybridization is the merging of an s orbital with two p orbitals.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
47
A carbon atom with two ð bonds and two ó bonds is sp2 hybridized.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
48
Electronegativity is used to determine the polarity of a bond.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
49
ó* represents an anti-bonding molecular orbital.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following structures is NOT breaking the octet rule?
A)BF3
B)CCl3+
C)H3O+
D)PO43-
A)BF3
B)CCl3+
C)H3O+
D)PO43-

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
51
To form a ó bond,two atomic orbitals overlap to form a single molecular orbital.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
52
A ó molecular orbital contains out-of-phase overlap of atomic orbitals.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
53
According to molecular orbital theory,all bonds contain a bonding and an anti-bonding orbital.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
54
Only filled molecular orbitals contribute to bonding.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
55
Anti-bonding orbitals are lower in energy than bonding orbitals.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
56
Resonance structures contain delocalized electrons.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
57
An sp3 hybridized atom has a tetrahedral geometry.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
58
An sp2 hybridized atom has a trigonal pyramidal geometry.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the labelled carbons in the molecule shown below is the most electron rich and which is the most electron deficient? 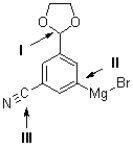
A)I is the most electron rich;II is the most electron deficient.
B)II is the most electron rich;I is the most electron deficient.
C)I is the most electron rich;III is the most electron deficient.
D)III is the most electron rich;I is the most electron deficient.

A)I is the most electron rich;II is the most electron deficient.
B)II is the most electron rich;I is the most electron deficient.
C)I is the most electron rich;III is the most electron deficient.
D)III is the most electron rich;I is the most electron deficient.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
60
The resonance hybrid is the most stable resonance form of a compound.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
61
Overlap of p orbitals is known as a _______________ bond.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
62
The two ð bonds in an triple bond are 180º from each other.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
63
A triple bond contains three ð bonds.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
64
An sp hybridized carbon has an orbital geometry of _______________ .

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
65
Hybridized orbitals are capable of resonance.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
66
Empty p orbitals are incapable of contributing to resonance structures.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
67
Triple bonds are not capable of contributing to resonance.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
68
Assign non-zero formal charges to the following molecule. 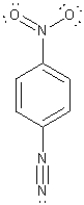


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
69
Anti-bonding orbitals involve out of plane overlap of atomic orbitals

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
70
A Nitrogen atom with four ó bonds has a _______________ formal charge.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
71
Assign non-zero formal charges and the hybridization to all atoms that are not hydrogen in the following molecule. 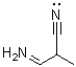


Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
72
A carbocation with three ó bonds is _______________ hybridized.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
73
Only carbon atoms can hybridize.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
74
Resonance requires atoms with neighbouring aligned p orbitals.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
75
Electrons shared among atoms are said to be _______________ .

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
76
An sp2 hybridized carbon has an orbital geometry of _______________ .

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
77
A carbocation with three ó bonds has a _______________ geometry.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
78
A carbon atom with two ð bonds and two ó bonds is _______________ hybridized.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
79
An sp hybridized atom has a _______________ angle between each electron group.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
80
The combined form of all resonance structures is referred to as the_______________.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck



