Deck 14: Chemical Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/123
Play
Full screen (f)
Deck 14: Chemical Equilibrium
1
The equilibrium constant is given for one of two reactions below. Determine the value of the missing equilibrium constant. N2O4(g)⇌ 2NO2(g)K = 1.46
3N2O4(g)⇌ 6NO2(g)K = ?
A)2.13
B)0.685
C)3.11
D)1.46
E)1.13
3N2O4(g)⇌ 6NO2(g)K = ?
A)2.13
B)0.685
C)3.11
D)1.46
E)1.13
3.11
2
The equilibrium constant is given for one of two reactions below. Determine the value of the missing equilibrium constant. H2(g)+ Br2(g)⇌ 2HBr(g)K = 3.8 × 104
2HBr(g)⇌ H2(g)+ Br2(g)K = ?
A)1.9 × 104
B)5.3 × 10-5
C)2.6 × 10-5
D)6.4 × 10-4
E)1.6 × 103
2HBr(g)⇌ H2(g)+ Br2(g)K = ?
A)1.9 × 104
B)5.3 × 10-5
C)2.6 × 10-5
D)6.4 × 10-4
E)1.6 × 103
2.6 × 10-5
3
The equilibrium constant is given for one of two reactions below. Determine the value of the missing equilibrium constant. H2(g)+ Br2(g)⇌ 2HBr(g)K = 3.8 × 104
4HBr(g)⇌ 2H2(g)+ 2Br2(g)K = ?
A)1.9 × 104
B)5.1 × 10-3
C)2.6 × 10-5
D)6.9 × 10-10
E)1.6 × 103
4HBr(g)⇌ 2H2(g)+ 2Br2(g)K = ?
A)1.9 × 104
B)5.1 × 10-3
C)2.6 × 10-5
D)6.9 × 10-10
E)1.6 × 103
6.9 × 10-10
4
For the following reaction, what is Δn required in the conversion of Kc to Kp? N2(g)+ 3H2(g)⇌ 2NH3(g)
A)4
B)-4
C)-2
D)2
E)1
A)4
B)-4
C)-2
D)2
E)1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
5
Express the equilibrium constant for the following reaction: CH3Cl(g)+ 1/2 Cl2(g)⇔ CH2Cl2(g)+ 1/2 H2(g)
A)KP =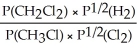
B)KP =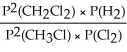
C)KP =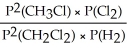
D)KP =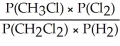
E)KP =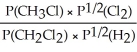
A)KP =
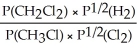
B)KP =
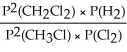
C)KP =
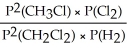
D)KP =
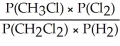
E)KP =
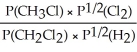

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements is correct?
A)When K >> 1, the reverse reaction is favoured and essentially goes to completion.
B)When K << 1, the forward reaction is favoured and the reverse reaction does not proceed to a great extent.
C)When K ≈ 1, neither the forward nor reverse reaction is strongly favoured, and about the same amount of reactants and products exist at equilibrium.
D)K >> 1 implies that the reaction is very fast at producing products.
E)When K = 0 the reaction is in equilibrium.
A)When K >> 1, the reverse reaction is favoured and essentially goes to completion.
B)When K << 1, the forward reaction is favoured and the reverse reaction does not proceed to a great extent.
C)When K ≈ 1, neither the forward nor reverse reaction is strongly favoured, and about the same amount of reactants and products exist at equilibrium.
D)K >> 1 implies that the reaction is very fast at producing products.
E)When K = 0 the reaction is in equilibrium.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
7
The equilibrium constant is given for two of the reactions below. Determine the value of the missing equilibrium constant. A(g)+ 2B(g)⇌ AB2(g) Kc = 59
AB2(g)+ B(g)⇌ AB3(g)Kc = ?
A(g)+ 3B(g)⇌ AB3(g)Kc = 478
A)3.5 × 10-5
B)2.8 × 104
C)8.1
D)0.12
E)89
AB2(g)+ B(g)⇌ AB3(g)Kc = ?
A(g)+ 3B(g)⇌ AB3(g)Kc = 478
A)3.5 × 10-5
B)2.8 × 104
C)8.1
D)0.12
E)89

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
8
Express the equilibrium constant for the following reaction: 2NH3(g)⇔ N2(g)+ 3H2(g)
A)KP =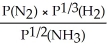
B)KP =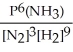
C)KP =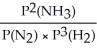
D)KP =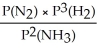
E)KP =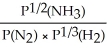
A)KP =
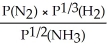
B)KP =
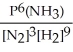
C)KP =
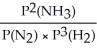
D)KP =
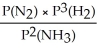
E)KP =
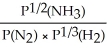

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
9
Express the equilibrium constant for the following reaction: N2(g)+ 3H2(g)⇔ 2NH3(g)
A)KP =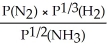
B)KP =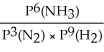
C)KP =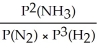
D)KP=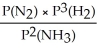
E)KP =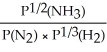
A)KP =
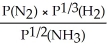
B)KP =
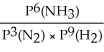
C)KP =
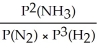
D)KP=
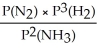
E)KP =
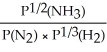

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
10
The equilibrium constant is given for one of two reactions below. Determine the value of the missing equilibrium constant. N2O4(g)⇌ 2NO2(g)K = 1.46
2N2O4(g)⇌ 4NO2(g)K = ?
A)2.13
B)0.685
C)3.11
D)1.46
E)1.13
2N2O4(g)⇌ 4NO2(g)K = ?
A)2.13
B)0.685
C)3.11
D)1.46
E)1.13

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
11
The equilibrium constant is given for two of the reactions below. Determine the value of the missing equilibrium constant. A(g)+ B(g)⇌ AB(g) K = 0.24
AB(g)+ A(g)⇌ A2B(g)K = 3.8
2A(g)+ B(g)⇌ A2B(g)K = ?
A)4.0
B)0.91
C)3.6
D)16
E)0.63
AB(g)+ A(g)⇌ A2B(g)K = 3.8
2A(g)+ B(g)⇌ A2B(g)K = ?
A)4.0
B)0.91
C)3.6
D)16
E)0.63

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
12
The equilibrium constant is given for one of two reactions below. Determine the value of the missing equilibrium constant. 2SO2(g)+ O2(g)⇌ 2SO3(g)K = 1.7 × 106
SO3(g)⇌ 1/2 O2(g)+ SO2(g)K = ?
A)3.4 × 102
B)8.5
C)1.3 × 103
D)1.2 × 10-6
E)7.7 × 10-4
SO3(g)⇌ 1/2 O2(g)+ SO2(g)K = ?
A)3.4 × 102
B)8.5
C)1.3 × 103
D)1.2 × 10-6
E)7.7 × 10-4

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
13
Express the equilibrium constant for the following reaction: 2P(g)+ 3Cl2(g)⇌ 2PCl3(g)
A)KP =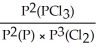
B)KP =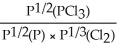
C)KP =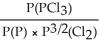
D)KP =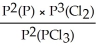
E)KP =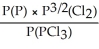
A)KP =
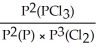
B)KP =
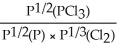
C)KP =
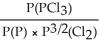
D)KP =
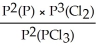
E)KP =
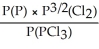

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
14
Express the equilibrium constant for the following reaction: H2(g)+ Br2(g)⇌ 2HBr(g)
A)KP =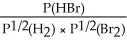
B)KP =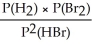
C)KP =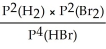
D)KP =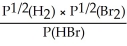
E)KP =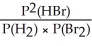
A)KP =
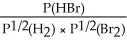
B)KP =
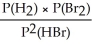
C)KP =
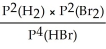
D)KP =
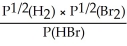
E)KP =
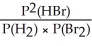

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
15
Express the equilibrium constant for the following reaction: P(g)+ 3/2 Cl2(g)⇌ PCl3(g)
A)KP =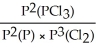
B)KP =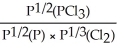
C)KP =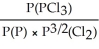
D)KP =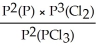
E)Kp =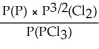
A)KP =
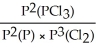
B)KP =
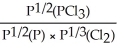
C)KP =
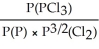
D)KP =
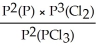
E)Kp =
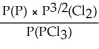

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
16
Express the equilibrium constant for the following reaction: PCl5(g)⇌ PCl3(g)+ Cl2(g)
A)KP =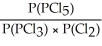
B)KP =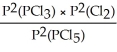
C)KP =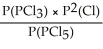
D)KP =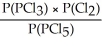
E)KP =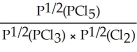
A)KP =
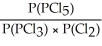
B)KP =
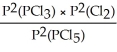
C)KP =
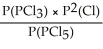
D)KP =
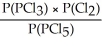
E)KP =
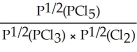

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
17
Give the direction of the reaction if K << 1.
A)The forward reaction is favoured.
B)The reverse reaction is favoured.
C)Neither direction is favoured.
D)If the temperature is raised, then the forward reaction is favoured.
E)If the temperature is raised, then the reverse reaction is favoured.
A)The forward reaction is favoured.
B)The reverse reaction is favoured.
C)Neither direction is favoured.
D)If the temperature is raised, then the forward reaction is favoured.
E)If the temperature is raised, then the reverse reaction is favoured.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
18
Give the direction of the reaction if K >> 1.
A)The forward reaction is favoured.
B)The reverse reaction is favoured.
C)Neither direction is favoured.
D)If the temperature is raised, then the forward reaction is favoured.
E)If the temperature is raised, then the reverse reaction is favoured.
A)The forward reaction is favoured.
B)The reverse reaction is favoured.
C)Neither direction is favoured.
D)If the temperature is raised, then the forward reaction is favoured.
E)If the temperature is raised, then the reverse reaction is favoured.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
19
The equilibrium constant is given for one of two reactions below. Determine the value of the missing equilibrium constant. 2HD(g)⇌ H2(g)+ D2(g) K = 0.28
2H2(g)+ 2D2(g)⇌ 4HD(g)K = ?
A)7.8 × 10-2
B)3.6
C)0.53
D)13
E)1.9
2H2(g)+ 2D2(g)⇌ 4HD(g)K = ?
A)7.8 × 10-2
B)3.6
C)0.53
D)13
E)1.9

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
20
Express the equilibrium constant for the following reaction: 2 CH3Cl(g)+ Cl2(g)⇔ 2 CH2Cl2(g)+ H2(g)
A)Kp =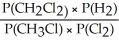
B)KP =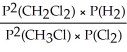
C)KP =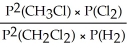
D)KP =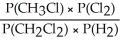
E)KP =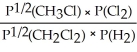
A)Kp =
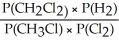
B)KP =
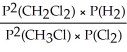
C)KP =
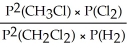
D)KP =
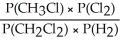
E)KP =
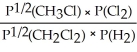

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
21
For which reaction will Kp = Kc?
A)S(s)+ O2(g)⇌ SO2(g)
B)2HgO(s)⇌ Hg(l)+ O2(g)
C)CaCO3(s)⇌ CaO(s)+ CO2(g)
D)H2CO3(s)⇌ H2O(l)+ CO2(g)
E)2H2O(l)⇌ 2H2(g)+ O2(g)
A)S(s)+ O2(g)⇌ SO2(g)
B)2HgO(s)⇌ Hg(l)+ O2(g)
C)CaCO3(s)⇌ CaO(s)+ CO2(g)
D)H2CO3(s)⇌ H2O(l)+ CO2(g)
E)2H2O(l)⇌ 2H2(g)+ O2(g)

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
22
For the following reaction, what is Δn required in the conversion of Kc to Kp? 2SO2(g)+ O2(g)⇌ 2SO3(g)
A)3
B)-1
C)-2
D)2
E)1
A)3
B)-1
C)-2
D)2
E)1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
23
For the following reaction, what is Δn required in the conversion of Kc to Kp? N2O4(g)⇌ 2NO2(g)
A)3
B)-1
C)-2
D)2
E)1
A)3
B)-1
C)-2
D)2
E)1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
24
Express the equilibrium constant for the following reaction: B(OH)3(aq) + 2H2O(l)⇌ B(OH)3(OH)-(aq)+ H3O+(aq)
A)Kc =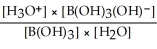
B)Kc =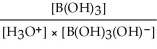
C)Kc =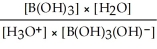
D)Kc =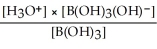
E)Kc =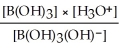
A)Kc =
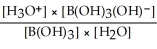
B)Kc =
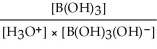
C)Kc =
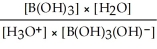
D)Kc =
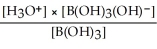
E)Kc =
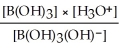

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
25
For which of the following reactions will Kc = Kp?
A)H2(g)+ I2(g)⇌ 2HI(g)
B)CH4(g)+ H2O(g)⇌ CO(g)+ 3H2(g)
C)N2O4(g)⇌ 2NO2(g)
D)CO(g)+ 2H2(g)⇌ CH3OH(g)
E)N2(g)+ 3H2(g)⇌ 2NH3(g)
A)H2(g)+ I2(g)⇌ 2HI(g)
B)CH4(g)+ H2O(g)⇌ CO(g)+ 3H2(g)
C)N2O4(g)⇌ 2NO2(g)
D)CO(g)+ 2H2(g)⇌ CH3OH(g)
E)N2(g)+ 3H2(g)⇌ 2NH3(g)

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
26
Express the equilibrium constant for the following reaction: KClO3(s)⇌ KClO(s)+ O2(g)
A)Kc =![<strong>Express the equilibrium constant for the following reaction: KClO<sub>3</sub>(s)⇌ KClO(s)+ O<sub>2</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [O<sub>2</sub>]<sup>-1</sup> C)K<sub>c</sub> = D)K<sub>c</sub> = E)K<sub>c</sub> = [O<sub>2</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_3a2a_8b3b_71117657f1a3_TB6103_11.jpg)
B)Kc = [O2]-1
C)Kc =![<strong>Express the equilibrium constant for the following reaction: KClO<sub>3</sub>(s)⇌ KClO(s)+ O<sub>2</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [O<sub>2</sub>]<sup>-1</sup> C)K<sub>c</sub> = D)K<sub>c</sub> = E)K<sub>c</sub> = [O<sub>2</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_613b_8b3b_7fd24bc20c69_TB6103_11.jpg)
D)Kc =![<strong>Express the equilibrium constant for the following reaction: KClO<sub>3</sub>(s)⇌ KClO(s)+ O<sub>2</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [O<sub>2</sub>]<sup>-1</sup> C)K<sub>c</sub> = D)K<sub>c</sub> = E)K<sub>c</sub> = [O<sub>2</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_613c_8b3b_3bd9366a08f7_TB6103_11.jpg)
E)Kc = [O2]
A)Kc =
![<strong>Express the equilibrium constant for the following reaction: KClO<sub>3</sub>(s)⇌ KClO(s)+ O<sub>2</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [O<sub>2</sub>]<sup>-1</sup> C)K<sub>c</sub> = D)K<sub>c</sub> = E)K<sub>c</sub> = [O<sub>2</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_3a2a_8b3b_71117657f1a3_TB6103_11.jpg)
B)Kc = [O2]-1
C)Kc =
![<strong>Express the equilibrium constant for the following reaction: KClO<sub>3</sub>(s)⇌ KClO(s)+ O<sub>2</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [O<sub>2</sub>]<sup>-1</sup> C)K<sub>c</sub> = D)K<sub>c</sub> = E)K<sub>c</sub> = [O<sub>2</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_613b_8b3b_7fd24bc20c69_TB6103_11.jpg)
D)Kc =
![<strong>Express the equilibrium constant for the following reaction: KClO<sub>3</sub>(s)⇌ KClO(s)+ O<sub>2</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [O<sub>2</sub>]<sup>-1</sup> C)K<sub>c</sub> = D)K<sub>c</sub> = E)K<sub>c</sub> = [O<sub>2</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_613c_8b3b_3bd9366a08f7_TB6103_11.jpg)
E)Kc = [O2]

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
27
For which of the following reactions will Kc = Kp?
A)4NH3(g)+ 3O2(g)⇌ 2N2(g)+ 6H2O(g)
B)SO3(g)+ NO(g)⇌ SO2(g)+ NO2(g)
C)2N2(g)+ O2(g)⇌ 2N2O(g)
D)2SO2(g)+ O2(g)⇌ 2SO3(g)
E)PCl3(g) + 1/2 O2(g) ⇌ PCl3O(g)
A)4NH3(g)+ 3O2(g)⇌ 2N2(g)+ 6H2O(g)
B)SO3(g)+ NO(g)⇌ SO2(g)+ NO2(g)
C)2N2(g)+ O2(g)⇌ 2N2O(g)
D)2SO2(g)+ O2(g)⇌ 2SO3(g)
E)PCl3(g) + 1/2 O2(g) ⇌ PCl3O(g)

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
28
The reaction below has a Kp value of 3.3 × 10-5 bar. What is the value of Kc for this reaction at 700 K? 2SO3(g)⇌ 2SO2(g)+ O2(g)
A)5.7 × 10-7 M
B)1.7 × 106 M
C)3.3 × 10-5 M
D)3.0 × 104 M
E)1.9 × 10-3 M
A)5.7 × 10-7 M
B)1.7 × 106 M
C)3.3 × 10-5 M
D)3.0 × 104 M
E)1.9 × 10-3 M

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
29
Express the equilibrium constant for the following reaction: P4(s)+ 5O2(g)⇌ P4O10(s)
A)Kc =![<strong>Express the equilibrium constant for the following reaction: P<sub>4</sub>(s)+ 5O<sub>2</sub>(g)⇌ P<sub>4</sub>O<sub>10</sub>(s)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = [O<sub>2</sub>]<sup>-5</sup> D)K<sub>c</sub> = [O<sub>2</sub>]<sup>5</sup> E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_1314_8b3b_f10931a58289_TB6103_11.jpg)
B)Kc =![<strong>Express the equilibrium constant for the following reaction: P<sub>4</sub>(s)+ 5O<sub>2</sub>(g)⇌ P<sub>4</sub>O<sub>10</sub>(s)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = [O<sub>2</sub>]<sup>-5</sup> D)K<sub>c</sub> = [O<sub>2</sub>]<sup>5</sup> E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_1315_8b3b_2fd9f9778206_TB6103_11.jpg)
C)Kc = [O2]-5
D)Kc = [O2]5
E)Kc =![<strong>Express the equilibrium constant for the following reaction: P<sub>4</sub>(s)+ 5O<sub>2</sub>(g)⇌ P<sub>4</sub>O<sub>10</sub>(s)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = [O<sub>2</sub>]<sup>-5</sup> D)K<sub>c</sub> = [O<sub>2</sub>]<sup>5</sup> E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_1316_8b3b_cd201afef5ba_TB6103_11.jpg)
A)Kc =
![<strong>Express the equilibrium constant for the following reaction: P<sub>4</sub>(s)+ 5O<sub>2</sub>(g)⇌ P<sub>4</sub>O<sub>10</sub>(s)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = [O<sub>2</sub>]<sup>-5</sup> D)K<sub>c</sub> = [O<sub>2</sub>]<sup>5</sup> E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_1314_8b3b_f10931a58289_TB6103_11.jpg)
B)Kc =
![<strong>Express the equilibrium constant for the following reaction: P<sub>4</sub>(s)+ 5O<sub>2</sub>(g)⇌ P<sub>4</sub>O<sub>10</sub>(s)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = [O<sub>2</sub>]<sup>-5</sup> D)K<sub>c</sub> = [O<sub>2</sub>]<sup>5</sup> E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_1315_8b3b_2fd9f9778206_TB6103_11.jpg)
C)Kc = [O2]-5
D)Kc = [O2]5
E)Kc =
![<strong>Express the equilibrium constant for the following reaction: P<sub>4</sub>(s)+ 5O<sub>2</sub>(g)⇌ P<sub>4</sub>O<sub>10</sub>(s)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = C)K<sub>c</sub> = [O<sub>2</sub>]<sup>-5</sup> D)K<sub>c</sub> = [O<sub>2</sub>]<sup>5</sup> E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_1316_8b3b_cd201afef5ba_TB6103_11.jpg)

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
30
For the following reaction, what is △n required in the conversion of Kc to Kp? KClO3(s)⇌ KClO(s)+ O2(g)
A)3
B)-1
C)-2
D)2
E)1
A)3
B)-1
C)-2
D)2
E)1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
31
Express the equilibrium constant for the following reaction: SO2(g) + H2O(l)⇌ H2SO3(aq)
A)K =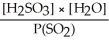
B)K =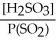
C)K =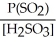
D)K =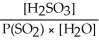
E)K =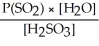
A)K =
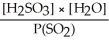
B)K =
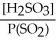
C)K =
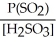
D)K =
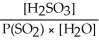
E)K =
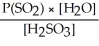

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
32
For the following reaction, what is Δn required in the conversion of Kc to Kp? CH4(g)+ H2O(g)⇌ CO(g)+ 3H2(g)
A)3
B)-1
C)-2
D)2
E)1
A)3
B)-1
C)-2
D)2
E)1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
33
For the following reaction, what is △n required in the conversion of Kc to Kp? 2Na(s)+ 2H2O(l)⇌ 2NaOH(aq)+ H2(g)
A)3
B)-1
C)-2
D)2
E)1
A)3
B)-1
C)-2
D)2
E)1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
34
Express the equilibrium constant for the following reaction: 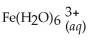 + 6CN-(aq)⇌
+ 6CN-(aq)⇌ 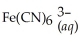 + 6H2O(l)
+ 6H2O(l)
A)Kc =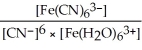
B)Kc =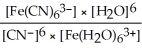
C)Kc =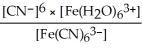
D)Kc =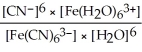
E)Kc =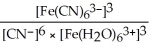
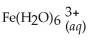 + 6CN-(aq)⇌
+ 6CN-(aq)⇌ 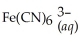 + 6H2O(l)
+ 6H2O(l)A)Kc =
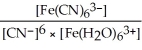
B)Kc =
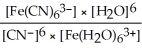
C)Kc =
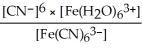
D)Kc =
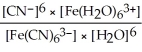
E)Kc =
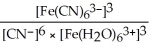

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
35
For the following reaction, what is △n required in the conversion of Kc to Kp? P4(s)+ 5O2(g)⇌ P4O10(s)
A)3
B)4
C)-4
D)5
E)-5
A)3
B)4
C)-4
D)5
E)-5

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
36
Express the equilibrium constant for the following reaction: 2Na(s)+ 2H2O(l)⇌ 2NaOH(aq)+ H2(g)
A)Kc =![<strong>Express the equilibrium constant for the following reaction: 2Na(s)+ 2H<sub>2</sub>O(l)⇌ 2NaOH(aq)+ H<sub>2</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [H<sub>2</sub>][NaOH]<sup>-2</sup> C)K<sub>c</sub> = D)K<sub>c</sub> = [H<sub>2</sub>][NaOH]<sup>2</sup> E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_3a27_8b3b_598f57eef15f_TB6103_11.jpg)
B)Kc = [H2][NaOH]-2
C)Kc =![<strong>Express the equilibrium constant for the following reaction: 2Na(s)+ 2H<sub>2</sub>O(l)⇌ 2NaOH(aq)+ H<sub>2</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [H<sub>2</sub>][NaOH]<sup>-2</sup> C)K<sub>c</sub> = D)K<sub>c</sub> = [H<sub>2</sub>][NaOH]<sup>2</sup> E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_3a28_8b3b_bb741f00a8ea_TB6103_11.jpg)
D)Kc = [H2][NaOH]2
E)Kc =![<strong>Express the equilibrium constant for the following reaction: 2Na(s)+ 2H<sub>2</sub>O(l)⇌ 2NaOH(aq)+ H<sub>2</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [H<sub>2</sub>][NaOH]<sup>-2</sup> C)K<sub>c</sub> = D)K<sub>c</sub> = [H<sub>2</sub>][NaOH]<sup>2</sup> E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_3a29_8b3b_89e27f4875fb_TB6103_11.jpg)
A)Kc =
![<strong>Express the equilibrium constant for the following reaction: 2Na(s)+ 2H<sub>2</sub>O(l)⇌ 2NaOH(aq)+ H<sub>2</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [H<sub>2</sub>][NaOH]<sup>-2</sup> C)K<sub>c</sub> = D)K<sub>c</sub> = [H<sub>2</sub>][NaOH]<sup>2</sup> E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_3a27_8b3b_598f57eef15f_TB6103_11.jpg)
B)Kc = [H2][NaOH]-2
C)Kc =
![<strong>Express the equilibrium constant for the following reaction: 2Na(s)+ 2H<sub>2</sub>O(l)⇌ 2NaOH(aq)+ H<sub>2</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [H<sub>2</sub>][NaOH]<sup>-2</sup> C)K<sub>c</sub> = D)K<sub>c</sub> = [H<sub>2</sub>][NaOH]<sup>2</sup> E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_3a28_8b3b_bb741f00a8ea_TB6103_11.jpg)
D)Kc = [H2][NaOH]2
E)Kc =
![<strong>Express the equilibrium constant for the following reaction: 2Na(s)+ 2H<sub>2</sub>O(l)⇌ 2NaOH(aq)+ H<sub>2</sub>(g)</strong> A)K<sub>c</sub> = B)K<sub>c</sub> = [H<sub>2</sub>][NaOH]<sup>-2</sup> C)K<sub>c</sub> = D)K<sub>c</sub> = [H<sub>2</sub>][NaOH]<sup>2</sup> E)K<sub>c</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB6103/11ea67b3_966f_3a29_8b3b_89e27f4875fb_TB6103_11.jpg)

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
37
Determine the value of Kc for the following reaction if the equilibrium concentrations are as follows: [N2]eq = 3.6 mol L-1, [O2]eq = 4.1 mol L-1, [N2O]eq = 3.3 × 10-18 mol L-1. 2N2(g)+ O2(g)⇌ 2N2O(g)
A)2.2 × 10-19
B)4.5 × 1018
C)2.0 × 10-37
D)5.0 × 1036
E)4.9 × 10-17
A)2.2 × 10-19
B)4.5 × 1018
C)2.0 × 10-37
D)5.0 × 1036
E)4.9 × 10-17

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
38
The reaction below has a Kc value of 1.0 × 1012 M-1. What is the value of Kp for this reaction at 500 K? 2SO2(g)+ O2(g)⇌ 2SO3(g)
A)4.2 × 10-11 bar-1
B)1.0 × 1012 bar-1
C)2.4 × 10-12 bar-1
D)4.1 × 1013 bar-1
E)2.4 × 1010 bar-1
A)4.2 × 10-11 bar-1
B)1.0 × 1012 bar-1
C)2.4 × 10-12 bar-1
D)4.1 × 1013 bar-1
E)2.4 × 1010 bar-1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
39
The reaction below has a Kc value of 61 M-2. What is the value of Kp for this reaction at 500 K? N2(g)+ 3H2(g)⇌ 2NH3(g)
A)15 bar-2
B)61 bar-2
C)28 bar-2
D)3.6 × 10-2 bar-2
E)1.9 × 10-2 bar-2
A)15 bar-2
B)61 bar-2
C)28 bar-2
D)3.6 × 10-2 bar-2
E)1.9 × 10-2 bar-2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
40
The reaction below has a Kp value of 41 bar-2. What is the value of Kc for this reaction at 400 K? N2(g)+ 3H2(g)⇌ 2NH3(g)
A)2.4 × 10-2 M-2
B)4.4 × 104 M-2
C)41 M-2
D)2.3 × 10-5 M-2
E)1.9 × 104 M-2
A)2.4 × 10-2 M-2
B)4.4 × 104 M-2
C)41 M-2
D)2.3 × 10-5 M-2
E)1.9 × 104 M-2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
41
Consider the following reaction and its equilibrium constant: I2(g)+ Br2(g)⇌ 2IBr(g)Kc = 1.1 × 102
A reaction mixture contains 0.41 mol L-1 I2, 0.27 mol L-1 Br2 and 3.5 mol L-1 IBr. Which of the following statements is TRUE concerning this system?
A)The reaction will shift in the direction of products.
B)The reaction will shift in the direction of reactants.
C)The reaction quotient will decrease.
D)The equilibrium constant will increase.
E)The system is at equilibrium.
A reaction mixture contains 0.41 mol L-1 I2, 0.27 mol L-1 Br2 and 3.5 mol L-1 IBr. Which of the following statements is TRUE concerning this system?
A)The reaction will shift in the direction of products.
B)The reaction will shift in the direction of reactants.
C)The reaction quotient will decrease.
D)The equilibrium constant will increase.
E)The system is at equilibrium.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the following reaction and its equilibrium constant: 4CuO(s)+ CH4(g)⇌ CO2(g)+ 4Cu(s)+ 2H2O(g)Kc = 1.10
A reaction mixture contains 0.22 mol L-1 CH4, 0.67 mol L-1 CO2 and 1.3 mol L-1 H2O. Which of the following statements is TRUE concerning this system?
A)The reaction will shift in the direction of products.
B)The equilibrium constant will increase.
C)The reaction quotient will increase.
D)The reaction will shift in the direction of reactants.
E)The system is at equilibrium.
A reaction mixture contains 0.22 mol L-1 CH4, 0.67 mol L-1 CO2 and 1.3 mol L-1 H2O. Which of the following statements is TRUE concerning this system?
A)The reaction will shift in the direction of products.
B)The equilibrium constant will increase.
C)The reaction quotient will increase.
D)The reaction will shift in the direction of reactants.
E)The system is at equilibrium.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
43
Determine the value of Kc for the following reaction if the equilibrium concentrations are as follows: [H2]eq = 0.14 mol L-1, [I2]eq = 0.39 mol L-1, [HI]eq = 1.6 mol L-1. H2(g)+ I2(g)⇌ 2HI(g)
A)2.1 × 10-2
B)29
C)47
D)3.4 × 10-2
E)8.7 × 10-2
A)2.1 × 10-2
B)29
C)47
D)3.4 × 10-2
E)8.7 × 10-2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
44
Consider the following reaction and its equilibrium constant: I2(g)⇌ 2I(g)Kp = 0.209 atm
A reaction mixture contains 0.89 atm I2 and 1.77 atm I. Which of the following statements is TRUE concerning this system?
A)The reaction will shift in the direction of reactants.
B)The reaction quotient will increase.
C)The reaction will shift in the direction of products.
D)The equilibrium constant will decrease.
E)The system is at equilibrium.
A reaction mixture contains 0.89 atm I2 and 1.77 atm I. Which of the following statements is TRUE concerning this system?
A)The reaction will shift in the direction of reactants.
B)The reaction quotient will increase.
C)The reaction will shift in the direction of products.
D)The equilibrium constant will decrease.
E)The system is at equilibrium.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following statements is TRUE?
A)If Q < K, it means the reverse reaction will proceed to form more reactants.
B)If Q > K, it means the forward reaction will proceed to form more products.
C)If Q = K, it means the reaction is at equilibrium.
D)If Q << K, it means the reaction is shifted to the left.
E)If Q >> K, it means the reaction is shifted to the right.
A)If Q < K, it means the reverse reaction will proceed to form more reactants.
B)If Q > K, it means the forward reaction will proceed to form more products.
C)If Q = K, it means the reaction is at equilibrium.
D)If Q << K, it means the reaction is shifted to the left.
E)If Q >> K, it means the reaction is shifted to the right.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
46
Calculate P (NO)eq, if P(NOCl)eq = 0.33 bar, P(Cl2)eq = 0.50 bar, and Kp = 1.9 × 10-2 bar . 2NOCl(g)⇌ 2NO(g)+ Cl2(g)
A)1.7 bar
B)0.0042 bar
C)0.30 bar
D)0.064 bar
E)0.087 bar
A)1.7 bar
B)0.0042 bar
C)0.30 bar
D)0.064 bar
E)0.087 bar

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
47
In a reaction mixture containing only reactants, what is the value of Q?
A)-1
B)1
C)∞
D)0
E)Q = K
A)-1
B)1
C)∞
D)0
E)Q = K

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
48
Determine the value of Kc for the following reaction if the equilibrium concentrations are as follows: [HCl]eq = 0.13 mol L-1, [HI]eq = 5.6 × 10-16 mol L-1, [Cl2]eq = 0.0019 mol L-1. 2HCl(g)+ I2(s)⇌ 2HI(g)+ Cl2(g)
A)8.2 × 10-18 M
B)2.9 × 1031 M
C)1.2 × 1017 M
D)1.4 × 10-19 M
E)3.5 × 10-32 M
A)8.2 × 10-18 M
B)2.9 × 1031 M
C)1.2 × 1017 M
D)1.4 × 10-19 M
E)3.5 × 10-32 M

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
49
Determine the value of Kp for the following reaction if the equilibrium concentrations are as follows: P(NOCl)eq = 0.22 atm, P(NO)eq = 0.10 atm, P(Cl2)eq = 0.081 atm. 2NOCl(g)⇌ 2NO(g)+ Cl2(g)
A)3.7 × 10-2 atm
B)60 atm
C)27 atm
D)1.7 × 10-2 atm
E)1.8 × 10-3 atm
A)3.7 × 10-2 atm
B)60 atm
C)27 atm
D)1.7 × 10-2 atm
E)1.8 × 10-3 atm

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
50
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particular temperature. Determine the equilibrium concentration of SO3(g). 2SO2(g)+ O2(g)⇌ 2SO3(g) Kc = 1.7 × 108 M-1
[SO2]eq = 0.0034 mol L-1 [O2]eq = 0.0018 mol L-1
A)1.9 mol L-1
B)1.0 × 103 mol L-1
C)0.53 mol L-1
D)9.6 × 10-4 mol L-1
E)0.73 mol L-1
[SO2]eq = 0.0034 mol L-1 [O2]eq = 0.0018 mol L-1
A)1.9 mol L-1
B)1.0 × 103 mol L-1
C)0.53 mol L-1
D)9.6 × 10-4 mol L-1
E)0.73 mol L-1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following statements is TRUE?
A)If Q < K, it means the reverse reaction will proceed to form more reactants.
B)If Q > K, it means the reverse reaction will proceed to form more reactants.
C)If Q = K, it means the reaction is not at equilibrium.
D)Because the equilibrium is always dynamic, Q can never be equal to K.
E)If Q < K, the reaction proceeding to the left is much faster than the reaction proceeding to the right.
A)If Q < K, it means the reverse reaction will proceed to form more reactants.
B)If Q > K, it means the reverse reaction will proceed to form more reactants.
C)If Q = K, it means the reaction is not at equilibrium.
D)Because the equilibrium is always dynamic, Q can never be equal to K.
E)If Q < K, the reaction proceeding to the left is much faster than the reaction proceeding to the right.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
52
Determine the value of Kc for the following reaction if the equilibrium concentrations are as follows: [N2]eq = 1.5 mol L-1, [H2]eq = 1.1 mol L-1, [NH3]eq = 0.47 mol L-1. N2(g)+ 3H2(g)⇌ 2NH3(g)
A)3.5 M-2
B)0.28 M-2
C)9.1 M-2
D)0.11 M-2
E)0.78 M-2
A)3.5 M-2
B)0.28 M-2
C)9.1 M-2
D)0.11 M-2
E)0.78 M-2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
53
In a reaction mixture containing only products, what is the value of Q?
A)-1
B)1
C)∞
D)0
E)Q = K
A)-1
B)1
C)∞
D)0
E)Q = K

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
54
In a reaction mixture containing reactants and products, each at a concentration of 1 mol L-1, what is the value of Q?
A)-1
B)1
C)∞
D)0
E)Q = K
A)-1
B)1
C)∞
D)0
E)Q = K

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
55
Consider the following reaction and its equilibrium constant: SO2(g)+ NO2(g)⇌ SO3(g)+ NO(g)Kc = 0.33
A reaction mixture contains 0.39 mol L-1 SO2, 0.14 mol L-1 NO2 , 0.11 mol L-1 SO3 and 0.14 mol L-1 NO. Which of the following statements is TRUE concerning this system?
A)The reaction will shift in the direction of reactants.
B)The equilibrium constant will decrease.
C)The reaction will shift in the direction of products.
D)The reaction quotient will decrease.
E)The system is at equilibrium.
A reaction mixture contains 0.39 mol L-1 SO2, 0.14 mol L-1 NO2 , 0.11 mol L-1 SO3 and 0.14 mol L-1 NO. Which of the following statements is TRUE concerning this system?
A)The reaction will shift in the direction of reactants.
B)The equilibrium constant will decrease.
C)The reaction will shift in the direction of products.
D)The reaction quotient will decrease.
E)The system is at equilibrium.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
56
Calculate the value of [N2]eq if [H2]eq = 2.0 mol L-1, [NH3]eq = 0.50 mol L-1, and Kc = 2.0. N2(g)+ 3H2(g)⇌ 2NH3(g)
A)0.016 mol L-1
B)0.031 mol L-1
C)0.062 mol L-1
D)0.40 mol L-1
E)62.5 mol L-1
A)0.016 mol L-1
B)0.031 mol L-1
C)0.062 mol L-1
D)0.40 mol L-1
E)62.5 mol L-1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
57
Determine the value of Kc for the following reaction if the equilibrium concentrations are as follows: [PCl5]eq = 0.56 mol L-1, [PCl3]eq = 0.23 mol L-1, [Cl2]eq = 4.4 mol L-1. PCl5(g)⇌ PCl3(g)+ Cl2(g)
A)1.8 M
B)0.93 M
C)0.55 M
D)1.1 M
E)0.76 M
A)1.8 M
B)0.93 M
C)0.55 M
D)1.1 M
E)0.76 M

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following statements is TRUE?
A)Dynamic equilibrium occurs when the rate of the forward reaction equals the rate of the reverse reaction.
B)The equilibrium constant for the forward reaction is equal to the equilibrium constant for the reverse reaction.
C)A reaction quotient (Q)larger than the equilibrium constant (K)means that the reaction will favour the production of more products.
D)Dynamic equilibrium indicates that the amount of reactants and products are equal.
E)Dynamic equilibrium is established when K = 0.
A)Dynamic equilibrium occurs when the rate of the forward reaction equals the rate of the reverse reaction.
B)The equilibrium constant for the forward reaction is equal to the equilibrium constant for the reverse reaction.
C)A reaction quotient (Q)larger than the equilibrium constant (K)means that the reaction will favour the production of more products.
D)Dynamic equilibrium indicates that the amount of reactants and products are equal.
E)Dynamic equilibrium is established when K = 0.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
59
Determine the value of Kp for the following reaction if the equilibrium concentrations are as follows: P(CO)eq = 6.8 × 10-11 atm, P(O2)eq = 1.3 × 10-3 atm, P(CO2)eq = 0.041 atm. 2 CO(g)+ O2(g)⇌ 2 CO2(g)
A)3.6 × 10-21 atm-1
B)2.8 × 1020 atm-1
C)4.6 × 1011 atm-1
D)2.2 × 10-12 atm-1
E)3.6 × 10-15 atm-1
A)3.6 × 10-21 atm-1
B)2.8 × 1020 atm-1
C)4.6 × 1011 atm-1
D)2.2 × 10-12 atm-1
E)3.6 × 10-15 atm-1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
60
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particular temperature. Determine the equilibrium concentration of H2O(g). C2H4(g)+ H2O(g)⇌ C2H5OH(g)Kc = 9.0 × 103 M-1
[C2H4]eq = 0.015 mol L-1 [C2H5OH]eq = 1.69 mol L-1
A)9.9 × 10-7 mol L-1
B)80. mol L-1
C)1.0 mol L-1
D)1.68 mol L-1
E)0.013 mol L-1
[C2H4]eq = 0.015 mol L-1 [C2H5OH]eq = 1.69 mol L-1
A)9.9 × 10-7 mol L-1
B)80. mol L-1
C)1.0 mol L-1
D)1.68 mol L-1
E)0.013 mol L-1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
61
The following reaction is exothermic. Which change will shift the equilibrium to the left? 2SO2(g)+ O2(g)⇌ 2SO3(g)
A)raising the temperature
B)removing SO3
C)adding O2
D)increasing the pressure
E)adding SO2
A)raising the temperature
B)removing SO3
C)adding O2
D)increasing the pressure
E)adding SO2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
62
Consider the following reaction at equilibrium. What effect will removing NO2 have on the system? SO2(g)+ NO2(g)⇌ SO3(g)+ NO(g)
A)The reaction will shift in the direction of products.
B)The reaction will shift to decrease the pressure.
C)No change will occur since NO2 is not included in the equilibrium expression.
D)The reaction will shift in the direction of reactants.
E)The equilibrium constant will decrease.
A)The reaction will shift in the direction of products.
B)The reaction will shift to decrease the pressure.
C)No change will occur since NO2 is not included in the equilibrium expression.
D)The reaction will shift in the direction of reactants.
E)The equilibrium constant will decrease.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
63
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particular temperature. Determine the equilibrium concentration of NO2(g). N2O4(g)⇌ 2NO2(g) Kc = 0.21 M
[N2O4]eq = 0.039 mol L-1
A)1.22 mol L-1
B)9.0 × 10-2 mol L-1
C)7.8 × 10-2 mol L-1
D)8.2 × 10-3 mol L-1
E)11 mol L-1
[N2O4]eq = 0.039 mol L-1
A)1.22 mol L-1
B)9.0 × 10-2 mol L-1
C)7.8 × 10-2 mol L-1
D)8.2 × 10-3 mol L-1
E)11 mol L-1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the following reaction at equilibrium. What effect will adding more H2S have on the system? 2H2S(g)+ 3O2(g)⇌ 2H2O(g)+ 2SO2(g)
A)The reaction will shift to the left.
B)No change will be observed.
C)The equilibrium constant will decrease.
D)The equilibrium constant will increase.
E)The reaction will shift in the direction of products.
A)The reaction will shift to the left.
B)No change will be observed.
C)The equilibrium constant will decrease.
D)The equilibrium constant will increase.
E)The reaction will shift in the direction of products.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
65
Consider the following reaction: CH4(g)+ 2H2S(g)⇌ CS2(g)+ 4H2(g)
A reaction mixture initially contains 0.50 mol L-1 CH4 and 0.75 mol L-1 H2S. If the equilibrium concentration of H2 is 0.44 mol L-1, find the equilibrium constant (mol L-1)for the reaction.
A)0.23 mol2 L-2
B)0.038 mol2 L-2
C)2.9 mol2 L-2
D)10. mol2 L-2
E)0.34 mol2 L-2
A reaction mixture initially contains 0.50 mol L-1 CH4 and 0.75 mol L-1 H2S. If the equilibrium concentration of H2 is 0.44 mol L-1, find the equilibrium constant (mol L-1)for the reaction.
A)0.23 mol2 L-2
B)0.038 mol2 L-2
C)2.9 mol2 L-2
D)10. mol2 L-2
E)0.34 mol2 L-2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
66
Identify the change that will always shift the equilibrium to the right.
A)remove reactant
B)increase product
C)remove product
D)increase pressure
E)increase volume
A)remove reactant
B)increase product
C)remove product
D)increase pressure
E)increase volume

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
67
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particular temperature. Determine the equilibrium concentration of CO2(g). NH2COONH4(s)⇌ 2NH3(g)+ CO2(g)Kc = 1.58 × 10-8 M3
[NH3]eq = 2.9 × 10-3 mol L-1
A)0.053 mol L-1
B)4.6 × 10-11 mol L-1
C)1.9 × 10-3 mol L-1
D)5.4 × 10-6 mol L-1
E)0.022 mol L-1
[NH3]eq = 2.9 × 10-3 mol L-1
A)0.053 mol L-1
B)4.6 × 10-11 mol L-1
C)1.9 × 10-3 mol L-1
D)5.4 × 10-6 mol L-1
E)0.022 mol L-1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
68
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particular temperature. Determine the equilibrium concentration of SO2(g). 2SO2(g)+ O2(g)⇌ 2SO3(g) Kc = 1.7 × 108 M-1
[SO3]eq = 0.0034 mol L-1 [O2]eq = 0.0018 mol L-1
A) 2.8 × 1013 mol L-1
B) 1.88 mol L-1
C)6.1 × 10-6 mol L-1
D)1.0 × 103 mol L-1
E)1.4 mol L-1
[SO3]eq = 0.0034 mol L-1 [O2]eq = 0.0018 mol L-1
A) 2.8 × 1013 mol L-1
B) 1.88 mol L-1
C)6.1 × 10-6 mol L-1
D)1.0 × 103 mol L-1
E)1.4 mol L-1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
69
Consider the following reaction at equilibrium. What effect will removing H2O have on the system? 2H2S(g)+ 3O2(g)⇌ 2H2O(g)+ 2SO2(g)
A)The reaction will shift to the left.
B)No change will be observed.
C)The equilibrium constant will decrease.
D)The equilibrium constant will increase.
E)The reaction will shift in the direction of products.
A)The reaction will shift to the left.
B)No change will be observed.
C)The equilibrium constant will decrease.
D)The equilibrium constant will increase.
E)The reaction will shift in the direction of products.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
70
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particular temperature. Determine the equilibrium pressure of H2. D2(g)+ H2(g)⇌ 2HD(g)Kp = 1.80
P(D2)eq = 1.1 × 10-3 bar P(HD)eq = 2.7 × 10-3 bar
A)2.7 bar
B)1.4 bar
C)0.73 bar
D)3.7 × 10-3 bar
E)8.1 × 10-4 bar
P(D2)eq = 1.1 × 10-3 bar P(HD)eq = 2.7 × 10-3 bar
A)2.7 bar
B)1.4 bar
C)0.73 bar
D)3.7 × 10-3 bar
E)8.1 × 10-4 bar

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
71
Consider the following reaction: CO2(g)+ C(graphite)⇌ 2CO(g)
A reaction mixture initially contains 0.56 bar CO2 and 0.32 bar CO. Determine the equilibrium pressure of CO if Kp for the reaction at this temperature is 2.25 bar.
A)0.83 bar
B)0.31 bar
C)0.26 bar
D)0.58 bar
E)0.42 bar
A reaction mixture initially contains 0.56 bar CO2 and 0.32 bar CO. Determine the equilibrium pressure of CO if Kp for the reaction at this temperature is 2.25 bar.
A)0.83 bar
B)0.31 bar
C)0.26 bar
D)0.58 bar
E)0.42 bar

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
72
Consider the following reaction at equilibrium. What effect will adding more SO3 have on the system? SO2(g)+ NO2(g)⇌ SO3(g)+ NO(g)
A)The reaction will shift in the direction of products.
B)The reaction will shift to decrease the pressure.
C)No change will occur since SO3 is not included in the equilibrium expression.
D)The reaction will shift in the direction of reactants.
E)The equilibrium constant will decrease.
A)The reaction will shift in the direction of products.
B)The reaction will shift to decrease the pressure.
C)No change will occur since SO3 is not included in the equilibrium expression.
D)The reaction will shift in the direction of reactants.
E)The equilibrium constant will decrease.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
73
The following reaction is exothermic. Which change will shift the equilibrium to the left? 2SO2(g)+ O2(g)⇌ 2SO3(g)
A)raising the temperature
B)adding a solid
C)adding argon
D)all of the above
E)none of the above
A)raising the temperature
B)adding a solid
C)adding argon
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
74
Consider the following reaction: Xe(g)+ 2F2(g)⇌ XeF4(g)
A reaction mixture initially contains 2.24 bar Xe and 4.27 bar F2. If the equilibrium pressure of Xe is 0.34 bar, find the equilibrium constant (Kp)for the reaction.
A)25 bar-2
B)0.12 bar-2
C)0.99 bar-2
D)8.3 bar-2
E)0.040 bar-2
A reaction mixture initially contains 2.24 bar Xe and 4.27 bar F2. If the equilibrium pressure of Xe is 0.34 bar, find the equilibrium constant (Kp)for the reaction.
A)25 bar-2
B)0.12 bar-2
C)0.99 bar-2
D)8.3 bar-2
E)0.040 bar-2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
75
Consider the following reaction: 2H2O(g)+ 2SO2(g)⇌ 2H2S(g)+ 3O2(g)
A reaction mixture initially contains 2.8 mol L-1 H2O and 2.6 mol L-1 SO2. Determine the equilibrium concentration of H2S if Kc for the reaction at this temperature is 1.3 × 10-6 mol L-1.
A)0.045 mol L-1
B)0.058 mol L-1
C)0.028 mol L-1
D)3.1 × 10-3 mol L-1
E)0.12 mol L-1
A reaction mixture initially contains 2.8 mol L-1 H2O and 2.6 mol L-1 SO2. Determine the equilibrium concentration of H2S if Kc for the reaction at this temperature is 1.3 × 10-6 mol L-1.
A)0.045 mol L-1
B)0.058 mol L-1
C)0.028 mol L-1
D)3.1 × 10-3 mol L-1
E)0.12 mol L-1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
76
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particular temperature. Determine the equilibrium pressure of CO. CO(g)+ 2H2(g)⇌ CH3OH(l)Kp = 2.25 × 104 bar-3
P(H2)eq = 0.52 bar
A)8.3 × 104 bar
B)1.2 × 10-5 bar
C)6.25 × 10-3 bar
D)8.5 × 10-5 bar
E)1.6 × 10-4 bar
P(H2)eq = 0.52 bar
A)8.3 × 104 bar
B)1.2 × 10-5 bar
C)6.25 × 10-3 bar
D)8.5 × 10-5 bar
E)1.6 × 10-4 bar

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
77
Consider the following reaction: COCl2(g)⇌ CO(g)+ Cl2(g)
A reaction mixture initially contains 1.6 mol L-1 COCl2. Determine the equilibrium concentration of CO if Kc for the reaction at this temperature is 8.33 × 10-4 M. Calculate this based on the assumption that the answer is negligible compared to 1.6.
A)4.2 × 10-4 mol L-1
B)1.5 × 10-3 mol L-1
C)3.7 × 10-2 mol L-1
D)2.1 × 10-2 mol L-1
E)1.3 × 10-3 mol L-1
A reaction mixture initially contains 1.6 mol L-1 COCl2. Determine the equilibrium concentration of CO if Kc for the reaction at this temperature is 8.33 × 10-4 M. Calculate this based on the assumption that the answer is negligible compared to 1.6.
A)4.2 × 10-4 mol L-1
B)1.5 × 10-3 mol L-1
C)3.7 × 10-2 mol L-1
D)2.1 × 10-2 mol L-1
E)1.3 × 10-3 mol L-1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
78
Consider the following reaction: CuS(s)+ O2(g)⇌ Cu(s)+ SO2(g)
A reaction mixture initially contains 2.9 mol L-1 O2. Determine the equilibrium concentration of O2 if Kc for the reaction at this temperature is 1.5.
A)1.9 mol L-1
B)1.7 mol L-1
C)2.2 mol L-1
D)1.2 mol L-1
E)0.59 mol L-1
A reaction mixture initially contains 2.9 mol L-1 O2. Determine the equilibrium concentration of O2 if Kc for the reaction at this temperature is 1.5.
A)1.9 mol L-1
B)1.7 mol L-1
C)2.2 mol L-1
D)1.2 mol L-1
E)0.59 mol L-1

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
79
The following reaction is exothermic. Which change will shift the equilibrium to the left? 2SO2(g)+ O2(g)⇌ 2SO3(g)
A)raising the temperature
B)decreasing the pressure
C)increasing the volume
D)all of the above
E)none of the above
A)raising the temperature
B)decreasing the pressure
C)increasing the volume
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
80
Consider the following reaction: NO(g)+ SO3(g)⇌ NO2(g)+ SO2(g)
A reaction mixture initially contains 0.86 bar NO and 0.86 bar SO3. Determine the equilibrium pressure of NO2 if Kp for the reaction at this temperature is 0.0118.
A)0.78 bar
B)0.084 bar
C)0.012 bar
D)0.85 bar
E)0.048 bar
A reaction mixture initially contains 0.86 bar NO and 0.86 bar SO3. Determine the equilibrium pressure of NO2 if Kp for the reaction at this temperature is 0.0118.
A)0.78 bar
B)0.084 bar
C)0.012 bar
D)0.85 bar
E)0.048 bar

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck



