Deck 23: Chemistry of the Nonmetals
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/50
Play
Full screen (f)
Deck 23: Chemistry of the Nonmetals
1
Determine the formula for an aluminosilicate where one-third of the silicons are replaced by an aluminum atom. The charge is balanced by potassium ions.
A)K2(AlO2)(SiO2)2
B)K(AlO2)(SiO2)
C)K(AlO2)2(SiO2)2
D)K(AlO2)(SiO2)2
E)K2(AlO2)2(SiO2)2
A)K2(AlO2)(SiO2)2
B)K(AlO2)(SiO2)
C)K(AlO2)2(SiO2)2
D)K(AlO2)(SiO2)2
E)K2(AlO2)2(SiO2)2
K(AlO2)(SiO2)2
2
Which of the following statements is TRUE?
A)All silicates are composed of SiO4 tetrahedra that bond together through an oxygen to form single chains.
B)The fibrous nature of asbestos comes from its silicate network structure.
C)In some silicates, ionic bonding between a metal ion and an SixOyz- ion is what holds the structure together.
D)The flakiness of mica comes from its single silicate chain structure.
E)Silicates are easily soluble in water because they contain silicate anions in their structure.
A)All silicates are composed of SiO4 tetrahedra that bond together through an oxygen to form single chains.
B)The fibrous nature of asbestos comes from its silicate network structure.
C)In some silicates, ionic bonding between a metal ion and an SixOyz- ion is what holds the structure together.
D)The flakiness of mica comes from its single silicate chain structure.
E)Silicates are easily soluble in water because they contain silicate anions in their structure.
In some silicates, ionic bonding between a metal ion and an SixOyz- ion is what holds the structure together.
3
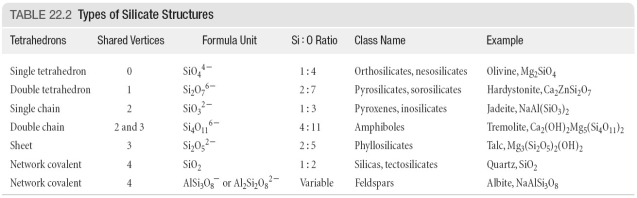
Refer to the above table. Use charge balance to determine the value of x in the pyrosilicate ZnxSi2O7.
A)1
B)3
C)6
D)4
E)2
3
4
Determine the formula for an aluminosilicate where one-quarter of the silicons are replaced by an aluminum atom. The charge is balanced by sodium ions.
A)Na2(AlO2)2(SiO2)
B)Na2(AlO2)2(SiO2)3
C)Na(AlO2)2(SiO2)
D)Na2(AlO2)2(SiO2)2
E)Na(AlO2)(SiO2)3
A)Na2(AlO2)2(SiO2)
B)Na2(AlO2)2(SiO2)3
C)Na(AlO2)2(SiO2)
D)Na2(AlO2)2(SiO2)2
E)Na(AlO2)(SiO2)3

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements is TRUE?
A)Boron oxide, when added to silicon oxide glass, forms a more heat stable glass called Pyrex.
B)Boron most commonly forms compounds that fulfill the octet rule.
C)Boron forms fairly weak bonds with oxygen because of their large electronegativity difference.
D)Boron trichloride is an example of a strong Lewis base.
E)Boron is a typical gaseous nonmetallic element.
A)Boron oxide, when added to silicon oxide glass, forms a more heat stable glass called Pyrex.
B)Boron most commonly forms compounds that fulfill the octet rule.
C)Boron forms fairly weak bonds with oxygen because of their large electronegativity difference.
D)Boron trichloride is an example of a strong Lewis base.
E)Boron is a typical gaseous nonmetallic element.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
6
Determine the formula for an aluminosilicate where one-half of the silicons are replaced by an aluminum atom. The charge is balanced by magnesium ions.
A)Mg(AlO2)2(SiO2)2
B)Mg2(AlO2)2(SiO2)2
C)Mg2(AlO2)2(SiO2)
D)Mg2(AlO2)(SiO2)2
E)Mg(AlO2)(SiO2)2
A)Mg(AlO2)2(SiO2)2
B)Mg2(AlO2)2(SiO2)2
C)Mg2(AlO2)2(SiO2)
D)Mg2(AlO2)(SiO2)2
E)Mg(AlO2)(SiO2)2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
7

Refer to the above table. Use charge balance to determine the value of x in the amphibole Mg2(OH)2Cax(Si4O11)2.
A)1
B)4
C)2
D)5
E)3

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
8
What is the shape of a boron trihalide?
A)trigonal planar
B)tetrahedral
C)linear
D)trigonal pyramidal
E)bent
A)trigonal planar
B)tetrahedral
C)linear
D)trigonal pyramidal
E)bent

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
9

Refer to the above table. Use charge balance to determine the value of x in the pyroxene Cax(SiO3)2.
A)2
B)1
C)4
D)6
E)3

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
10
Determine the number of vertices and faces in the closo-Borane B7H72-.
A)vertices = 4, faces = 10
B)vertices = 7, faces = 14
C)vertices = 3, faces = 6
D)vertices = 14, faces = 24
E)vertices = 7, faces = 10
A)vertices = 4, faces = 10
B)vertices = 7, faces = 14
C)vertices = 3, faces = 6
D)vertices = 14, faces = 24
E)vertices = 7, faces = 10

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
11

Refer to the above table. Use charge balance to determine the value of x in the orthosilicate CaxSiO4.
A)3
B)1
C)2
D)4
E)6

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following are major components of Earth's crust?
A)oxygen and silicon
B)calcium and silicon
C)oxygen and iron
D)oxygen, hydrogen, and carbon
E)oxygen, iron, and carbon
A)oxygen and silicon
B)calcium and silicon
C)oxygen and iron
D)oxygen, hydrogen, and carbon
E)oxygen, iron, and carbon

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements is TRUE?
A)Silicon atoms form π bonds as easily as carbon atoms do.
B)Silicates are covalent atomic solids that contain silicon and oxygen.
C)Silicon is the most abundant element in the Earth's crust.
D)Silicon is one of the strongest oxidizing agents.
E)SIlicon is the most electronegative element.
A)Silicon atoms form π bonds as easily as carbon atoms do.
B)Silicates are covalent atomic solids that contain silicon and oxygen.
C)Silicon is the most abundant element in the Earth's crust.
D)Silicon is one of the strongest oxidizing agents.
E)SIlicon is the most electronegative element.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements is TRUE?
A)Boron is a large component of Earth's crust.
B)Boron is usually found in its elemental state in the Earth's crust.
C)Due to boron's small size and low electronegativity, it behaves as a semimetal instead of a metal.
D)Boron atoms don't typically bond to one another due to its small atomic radius.
E)There are at least five allotropes of elemental boron.
A)Boron is a large component of Earth's crust.
B)Boron is usually found in its elemental state in the Earth's crust.
C)Due to boron's small size and low electronegativity, it behaves as a semimetal instead of a metal.
D)Boron atoms don't typically bond to one another due to its small atomic radius.
E)There are at least five allotropes of elemental boron.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
15

Refer to the above table. Predict the silicate structure for KAl(SiO3)2.
A)phyllosilicate
B)orthosilicate
C)amphibole
D)pyroxene
E)pyrosilicate

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
16

Refer to the above table. Predict the silicate structure for NaAl(SiO4).
A)pyrosilicate
B)amphibole
C)orthosilicate
D)phyllosilicate
E)pyroxene

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
17
Determine the number of vertices and faces in the closo-Borane B10H102-.
A)vertices = 10, faces = 16
B)vertices = 5, faces = 6
C)vertices = 5, faces = 10
D)vertices = 10, faces = 20
E)vertices = 20, faces = 36
A)vertices = 10, faces = 16
B)vertices = 5, faces = 6
C)vertices = 5, faces = 10
D)vertices = 10, faces = 20
E)vertices = 20, faces = 36

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
18
Identify the formula of the compound that is in common glass.
A)SiO2
B)Si2O3
C)SiO3
D)Si2O2
E)SiO4
A)SiO2
B)Si2O3
C)SiO3
D)Si2O2
E)SiO4

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
19

Refer to the above table. Use charge balance to determine the value of x in the phyllosilicate Ca3(Si2O5)2(OH)x.
A)6
B)4
C)3
D)1
E)2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
20

Refer to the above table. Predict the silicate structure for CaMg2(Si2O7).
A)orthosilicate
B)pyrosilicate
C)pyroxene
D)amphibole
E)phyllosilicate

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
21
Determine the oxidation state of sulfur in S2O32-.
A)+3
B)+6
C)+2
D)+4
E)0
A)+3
B)+6
C)+2
D)+4
E)0

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
22
Determine the oxidation state of carbon in the carbonate ion CO32-.
A)+2
B)+4
C)+3
D)+6
E)+8
A)+2
B)+4
C)+3
D)+6
E)+8

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following statements is TRUE?
A)Sheets of graphitic carbon bond strongly to one another.
B)The graphite form of carbon is more dense than diamond.
C)Carbon atoms sometimes cluster together into substances called fullerenes.
D)The diamond form of carbon conducts electricity.
E)Graphite is the hardest known material.
A)Sheets of graphitic carbon bond strongly to one another.
B)The graphite form of carbon is more dense than diamond.
C)Carbon atoms sometimes cluster together into substances called fullerenes.
D)The diamond form of carbon conducts electricity.
E)Graphite is the hardest known material.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following statements is TRUE?
A)Water and H2S have the same bond angles.
B)Hydrofluoric acid is a strong reducing agent.
C)Sulfur is a stronger oxidizing agent than oxygen.
D)Water is less stable than H2S.
E)Sulfuric acid is a strong oxidizing agent.
A)Water and H2S have the same bond angles.
B)Hydrofluoric acid is a strong reducing agent.
C)Sulfur is a stronger oxidizing agent than oxygen.
D)Water is less stable than H2S.
E)Sulfuric acid is a strong oxidizing agent.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
25
Determine the oxidation state of carbon in the ionic carbide CaC2.
A)-2
B)+4
C)-1
D)+1
E)-4
A)-2
B)+4
C)-1
D)+1
E)-4

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
26
Determine the oxidation state of nitrogen in NO3⁻.
A)+1
B)+6
C)+2
D)+3
E)+5
A)+1
B)+6
C)+2
D)+3
E)+5

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following statements is TRUE?
A)Ammonium nitrate is insoluble in water.
B)Sodium nitrite is used in the glass industry.
C)NO2 reacts with water to form ammonia.
D)Hydrazine is a good reducing agent.
E)Nitric acid is a weak acid.
A)Ammonium nitrate is insoluble in water.
B)Sodium nitrite is used in the glass industry.
C)NO2 reacts with water to form ammonia.
D)Hydrazine is a good reducing agent.
E)Nitric acid is a weak acid.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
28
What is the name of powdered form of carbon, which is a component of soot?
A)diamond
B)graphite
C)fullerenes
D)bucky balls
E)carbon black
A)diamond
B)graphite
C)fullerenes
D)bucky balls
E)carbon black

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
29
Determine the number of vertices and faces in the closo-Borane B12H122-.
A)vertices = 12, faces = 24
B)vertices = 12, faces = 20
C)vertices = 8, faces = 16
D)vertices = 6, faces = 8
E)vertices = 8, faces = 12
A)vertices = 12, faces = 24
B)vertices = 12, faces = 20
C)vertices = 8, faces = 16
D)vertices = 6, faces = 8
E)vertices = 8, faces = 12

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
30
Determine the oxidation state of nitrogen in NaN3.
A)+1
B)+3
C)-1
D)-
E)-3
A)+1
B)+3
C)-1
D)-

E)-3

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
31
What is the oxidation number of oxygen in Na2O?
A)-4
B)+2
C)-2
D)-1
E)+1
A)-4
B)+2
C)-2
D)-1
E)+1

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
32
Determine the oxidation state of phosphorus in P2O74⁻.
A)+5
B)+4
C)+3
D)+7
E)+2
A)+5
B)+4
C)+3
D)+7
E)+2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
33
What is the major drawback to using bituminous coal as an energy source?
A)It has very low carbon content.
B)It contains a high amount of sulfur, which when burned contributes to acid rain.
C)It has an extremely high surface area, which makes it difficult to burn.
D)Bituminous coal is noncrystalline, which gives it a low energy/gram ratio.
E)Bituminous coal is very expensive to produce and transport.
A)It has very low carbon content.
B)It contains a high amount of sulfur, which when burned contributes to acid rain.
C)It has an extremely high surface area, which makes it difficult to burn.
D)Bituminous coal is noncrystalline, which gives it a low energy/gram ratio.
E)Bituminous coal is very expensive to produce and transport.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
34
Determine the oxidation state of chlorine in ClO⁻.
A)0
B)+1
C)-2
D)-1
E)+2
A)0
B)+1
C)-2
D)-1
E)+2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
35
What is the oxidation number of oxygen in H2O2?
A)-4
B)+2
C)-2
D)-1
E)+1
A)-4
B)+2
C)-2
D)-1
E)+1

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following statements is TRUE?
A)Ionic carbides form between carbon and another highly electronegative element.
B)Carbides are soft materials with low melting points.
C)Metallic carbides are more malleable than the pure metal.
D)Covalent carbides are formed between carbon and a nonmetal with low electronegativity.
E)Carbides can be cationic, anionic, and covalent.
A)Ionic carbides form between carbon and another highly electronegative element.
B)Carbides are soft materials with low melting points.
C)Metallic carbides are more malleable than the pure metal.
D)Covalent carbides are formed between carbon and a nonmetal with low electronegativity.
E)Carbides can be cationic, anionic, and covalent.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is TRUE?
A)Phosphoric acid is used to produce the "fizziness" in soda.
B)Sodium phosphate is an additive in baked goods to give them a longer shelf life.
C)Phosphorus can range in oxidation state from -3 to +5.
D)The phosphorus in the phosphite ion has an oxidation state of -3.
E)Phosphoric acid is a very strong oxidizing agent, just like nitric acid.
A)Phosphoric acid is used to produce the "fizziness" in soda.
B)Sodium phosphate is an additive in baked goods to give them a longer shelf life.
C)Phosphorus can range in oxidation state from -3 to +5.
D)The phosphorus in the phosphite ion has an oxidation state of -3.
E)Phosphoric acid is a very strong oxidizing agent, just like nitric acid.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
38
Tetraphosphorus decoxide reacts with water to produce phosphoric acid. Write a balanced reaction for this process.
A)P4O10 + 6H2O → 4H3PO4
B)P2O5 + 3H2O → 2H3PO3
C)P4 + 3O2 + 6H2O → 4H3PO4
D)P4O10 + 4H2O → 4H2PO3
E)P4 + 3O2 + 6H2O → 4H3PO3
A)P4O10 + 6H2O → 4H3PO4
B)P2O5 + 3H2O → 2H3PO3
C)P4 + 3O2 + 6H2O → 4H3PO4
D)P4O10 + 4H2O → 4H2PO3
E)P4 + 3O2 + 6H2O → 4H3PO3

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
39
Identify an example of a nitrite.
A)NaNO2
B)NO
C)N2O
D)NaNO3
E)NO2
A)NaNO2
B)NO
C)N2O
D)NaNO3
E)NO2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
40
Write the balanced equation that represents the formation of hydrofluoric acid from the reaction of calcium fluoride with sulfuric acid.
A)CaF2 + H2S → CaS + 2HF
B)CF4 + 2H2S → 4HF + CS2
C)CF4 + 2H2SO4 → 4HF + CS2 + CO2
D)CaF2 + H2SO4 → 2HF + CaSO4
E)CaF2 + H2SO3 → CaSO3 + 2HF
A)CaF2 + H2S → CaS + 2HF
B)CF4 + 2H2S → 4HF + CS2
C)CF4 + 2H2SO4 → 4HF + CS2 + CO2
D)CaF2 + H2SO4 → 2HF + CaSO4
E)CaF2 + H2SO3 → CaSO3 + 2HF

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
41
Match the following.
pyrosilicate
A)Si2O52-
B)SiO44-
C)Si4O116-
D)SiO32-
E)Si2O76-
pyrosilicate
A)Si2O52-
B)SiO44-
C)Si4O116-
D)SiO32-
E)Si2O76-

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
42
Draw the Lewis structure for IF4⁻ and determine the geometry of the ion.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
43
Write the balanced reaction that shows the formation of ICl3 from its elements.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
44
Why are phosphate compounds added to detergents?

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
45
Match the following.
pyroxene
A)Si2O52-
B)SiO44-
C)Si4O116-
D)SiO32-
E)Si2O76-
pyroxene
A)Si2O52-
B)SiO44-
C)Si4O116-
D)SiO32-
E)Si2O76-

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
46
Why are the chemical properties of nitrogen and phosphorus so different when they are in the same family?

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
47
Match the following.
amphibole
A)Si2O52-
B)SiO44-
C)Si4O116-
D)SiO32-
E)Si2O76-
amphibole
A)Si2O52-
B)SiO44-
C)Si4O116-
D)SiO32-
E)Si2O76-

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
48
Describe the major production method for obtaining oxygen.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
49
Match the following.
phyllosilicate
A)Si2O52-
B)SiO44-
C)Si4O116-
D)SiO32-
E)Si2O76-
phyllosilicate
A)Si2O52-
B)SiO44-
C)Si4O116-
D)SiO32-
E)Si2O76-

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
50
Match the following.
orthosilicate
A)Si2O52-
B)SiO44-
C)Si4O116-
D)SiO32-
E)Si2O76-
orthosilicate
A)Si2O52-
B)SiO44-
C)Si4O116-
D)SiO32-
E)Si2O76-

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck



