Deck 24: Metals and Metallury
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/49
Play
Full screen (f)
Deck 24: Metals and Metallury
1
Which of the following processes is used to form metal components from very small metal particles?
A)electrometallurgy
B)hydrometallurgy
C)pyrometallurgy
D)powder metallurgy
E)smelting
A)electrometallurgy
B)hydrometallurgy
C)pyrometallurgy
D)powder metallurgy
E)smelting
powder metallurgy
2
Identify the substances that are homogeneous, naturally occurring, crystalline inorganic solids.
A)slag
B)minerals
C)ores
D)gangue
E)alloy
A)slag
B)minerals
C)ores
D)gangue
E)alloy
minerals
3
Which of the following describes slag?
A)a waste material that is the product of smelting a metal ore
B)the aqueous portion of the gangue separated from a metal ore
C)a low-density waste product, formed from the reaction of gangue with another substance, that can be easily separated from the metal ore of interest
D)a substance that reacts with the gangue to make a low-density liquid compound that can be easily separated from the metal of interest
E)a heavy precipitate containing by-products obtained during the purification process
A)a waste material that is the product of smelting a metal ore
B)the aqueous portion of the gangue separated from a metal ore
C)a low-density waste product, formed from the reaction of gangue with another substance, that can be easily separated from the metal ore of interest
D)a substance that reacts with the gangue to make a low-density liquid compound that can be easily separated from the metal of interest
E)a heavy precipitate containing by-products obtained during the purification process
a low-density waste product, formed from the reaction of gangue with another substance, that can be easily separated from the metal ore of interest
4
Which of the following is an example of calcination?
A)2CuS(s)+ 3O2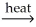 2CuO(s)+ 2SO2(g)
2CuO(s)+ 2SO2(g)
B)SnO2(s)+ 2C(s)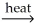 Sn(l)+ 2CO(g)
Sn(l)+ 2CO(g)
C)CaCO3(s)+ SiO2(s)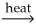 CaSiO3(l)+ CO2(g)
CaSiO3(l)+ CO2(g)
D)MgCO3(s)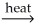 MgO(s)+ CO2(g)
MgO(s)+ CO2(g)
E)PbO(s)+ CO(g)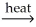 Pb(l)+ CO2(g)
Pb(l)+ CO2(g)
A)2CuS(s)+ 3O2
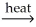 2CuO(s)+ 2SO2(g)
2CuO(s)+ 2SO2(g)B)SnO2(s)+ 2C(s)
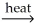 Sn(l)+ 2CO(g)
Sn(l)+ 2CO(g)C)CaCO3(s)+ SiO2(s)
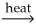 CaSiO3(l)+ CO2(g)
CaSiO3(l)+ CO2(g)D)MgCO3(s)
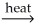 MgO(s)+ CO2(g)
MgO(s)+ CO2(g)E)PbO(s)+ CO(g)
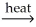 Pb(l)+ CO2(g)
Pb(l)+ CO2(g)
Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
5
Powder metallurgy is
A)refining of metal ores using oxidation-reduction reactions.
B)refining of metal ores using heat.
C)forming metal parts using electricity.
D)refining of metal ores using reactions with aqueous solutions.
E)forming metal parts using heat and small crystals of metal.
A)refining of metal ores using oxidation-reduction reactions.
B)refining of metal ores using heat.
C)forming metal parts using electricity.
D)refining of metal ores using reactions with aqueous solutions.
E)forming metal parts using heat and small crystals of metal.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
6
Leaching is
A)melting an ore and separating it from the lower density gangue.
B)selectively dissolving a metal in solution to separate it from its ore.
C)the process of heating an ore to drive off volatile compounds.
D)heating an ore in the presence of oxygen or another substance to purify the ore and obtain the liquid metal.
E)heating an ore in the presence of oxygen or another substance to cause a chemical reaction that drives off newly formed volatile compounds.
A)melting an ore and separating it from the lower density gangue.
B)selectively dissolving a metal in solution to separate it from its ore.
C)the process of heating an ore to drive off volatile compounds.
D)heating an ore in the presence of oxygen or another substance to purify the ore and obtain the liquid metal.
E)heating an ore in the presence of oxygen or another substance to cause a chemical reaction that drives off newly formed volatile compounds.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is an example of roasting?
A)CaCO3(s)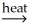 CaO(s)+ CO2(g)
CaO(s)+ CO2(g)
B)PbO(s)+ CO(g)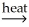 Pb(l)+ CO2(g)
Pb(l)+ CO2(g)
C)SnO2(s)+ 2C(s)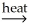 Sn(l)+ 2CO(g)
Sn(l)+ 2CO(g)
D)Ni(OH)2(s)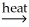 NiO(s)+ H2O(g)
NiO(s)+ H2O(g)
E)2CuS(s)+ 3O2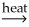 2CuO(s)+ 2SO2(g)
2CuO(s)+ 2SO2(g)
A)CaCO3(s)
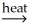 CaO(s)+ CO2(g)
CaO(s)+ CO2(g)B)PbO(s)+ CO(g)
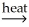 Pb(l)+ CO2(g)
Pb(l)+ CO2(g)C)SnO2(s)+ 2C(s)
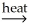 Sn(l)+ 2CO(g)
Sn(l)+ 2CO(g)D)Ni(OH)2(s)
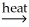 NiO(s)+ H2O(g)
NiO(s)+ H2O(g)E)2CuS(s)+ 3O2
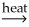 2CuO(s)+ 2SO2(g)
2CuO(s)+ 2SO2(g)
Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
8
Which compound in the following reaction is the slag? 2Ca3(PO4)2(s)+ 6SiO2(s)+ 10C(s) 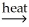 P4(g)+ 6CaSiO3(l)+ 10CO(s)
P4(g)+ 6CaSiO3(l)+ 10CO(s)
A)CaSiO3
B)CO
C)P4
D)SiO2
E)Ca3(PO4)2
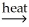 P4(g)+ 6CaSiO3(l)+ 10CO(s)
P4(g)+ 6CaSiO3(l)+ 10CO(s)A)CaSiO3
B)CO
C)P4
D)SiO2
E)Ca3(PO4)2

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is an example of smelting?
A)PbO(s)+ CO(g)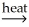 Pb(l)+ CO2(g)
Pb(l)+ CO2(g)
B)2CuS(s)+ 3O2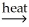 2CuO(s)+ 2SO2(g)
2CuO(s)+ 2SO2(g)
C)CaCO3(s)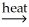 CaO(s)+ CO2(g)
CaO(s)+ CO2(g)
D)Ni(OH)2(s)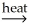 NiO(s)+ H2O(g)
NiO(s)+ H2O(g)
E)2Hg(l)+ O2(g)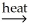 2HgO(s)
2HgO(s)
A)PbO(s)+ CO(g)
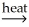 Pb(l)+ CO2(g)
Pb(l)+ CO2(g)B)2CuS(s)+ 3O2
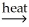 2CuO(s)+ 2SO2(g)
2CuO(s)+ 2SO2(g)C)CaCO3(s)
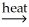 CaO(s)+ CO2(g)
CaO(s)+ CO2(g)D)Ni(OH)2(s)
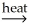 NiO(s)+ H2O(g)
NiO(s)+ H2O(g)E)2Hg(l)+ O2(g)
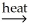 2HgO(s)
2HgO(s)
Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
10
Roasting is
A)forming metal parts using heat and small crystals of metal.
B)the process of heating an ore to drive off volatile compounds.
C)selectively dissolving a metal in solution to separate it from its ore.
D)heating an ore in the presence of oxygen or another substance to purify the ore and obtain the liquid metal.
E)heating an ore in the presence of oxygen or another substance to cause a chemical reaction that drives off newly formed volatile compounds.
A)forming metal parts using heat and small crystals of metal.
B)the process of heating an ore to drive off volatile compounds.
C)selectively dissolving a metal in solution to separate it from its ore.
D)heating an ore in the presence of oxygen or another substance to purify the ore and obtain the liquid metal.
E)heating an ore in the presence of oxygen or another substance to cause a chemical reaction that drives off newly formed volatile compounds.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
11
What method is used to obtain pure aluminum metal from Al2O3?
A)smelting
B)powder metallurgy
C)Hall process
D)leaching
E)calcination
A)smelting
B)powder metallurgy
C)Hall process
D)leaching
E)calcination

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
12
Smelting is
A)heating an ore in the presence of oxygen or another substance to purify the ore and obtain the liquid metal.
B)selectively dissolving a metal in solution to separate it from its ore.
C)the process of heating an ore to drive off volatile compounds.
D)heating an ore in the presence of oxygen or another substance to cause a chemical reaction that drives off newly formed volatile compounds.
E)forming metal parts using heat and small crystals of metal.
A)heating an ore in the presence of oxygen or another substance to purify the ore and obtain the liquid metal.
B)selectively dissolving a metal in solution to separate it from its ore.
C)the process of heating an ore to drive off volatile compounds.
D)heating an ore in the presence of oxygen or another substance to cause a chemical reaction that drives off newly formed volatile compounds.
E)forming metal parts using heat and small crystals of metal.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following describes gangue?
A)the undesirable product formed during smelting
B)the undesirable portion of a metal-containing ore
C)the volatile product formed during calcination
D)a waste material that is the product of smelting a metal ore
E)a crude metal that still has to be further purified for industrial use
A)the undesirable product formed during smelting
B)the undesirable portion of a metal-containing ore
C)the volatile product formed during calcination
D)a waste material that is the product of smelting a metal ore
E)a crude metal that still has to be further purified for industrial use

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
14
Electrometallurgy is
A)refining of metal ores using heat.
B)forming metal parts using electricity.
C)forming metal parts using heat and small crystals of metal.
D)refining of metal ores using oxidation-reduction reactions.
E)refining of metal ores using reactions with aqueous solutions.
A)refining of metal ores using heat.
B)forming metal parts using electricity.
C)forming metal parts using heat and small crystals of metal.
D)refining of metal ores using oxidation-reduction reactions.
E)refining of metal ores using reactions with aqueous solutions.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
15
Calcination is
A)heating an ore in the presence of oxygen or another substance to cause a chemical reaction that drives off newly formed volatile compounds.
B)heating an ore in the presence of oxygen or another substance to purify the ore and obtain the liquid metal.
C)selectively dissolving a metal in solution to separate it from its ore.
D)the process of heating an ore to drive off volatile compounds.
E)forming metal parts using heat and small crystals of metal.
A)heating an ore in the presence of oxygen or another substance to cause a chemical reaction that drives off newly formed volatile compounds.
B)heating an ore in the presence of oxygen or another substance to purify the ore and obtain the liquid metal.
C)selectively dissolving a metal in solution to separate it from its ore.
D)the process of heating an ore to drive off volatile compounds.
E)forming metal parts using heat and small crystals of metal.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a general property of a metal?
A)high resistivity
B)good conductor of heat
C)low boiling point
D)low melting point
E)high electronegativity
A)high resistivity
B)good conductor of heat
C)low boiling point
D)low melting point
E)high electronegativity

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
17
Pyrometallurgy is the
A)refining of metal ores using reactions with aqueous solutions.
B)refining of metal ores using heat.
C)refining of metal ores using oxidation-reduction reactions.
D)forming of metal parts using heat and small crystals of metal.
E)forming of metal parts using electricity.
A)refining of metal ores using reactions with aqueous solutions.
B)refining of metal ores using heat.
C)refining of metal ores using oxidation-reduction reactions.
D)forming of metal parts using heat and small crystals of metal.
E)forming of metal parts using electricity.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following describes flux?
A)a waste material that is the product of smelting a metal ore
B)the aqueous portion of the gangue separated from a metal ore
C)a low-density waste product, formed from the reaction of gangue with another substance, that can be easily separated from the metal ore of interest
D)a substance that reacts with the gangue to make a low-density liquid compound that can be easily separated from the metal of interest
E)a low-density material that floats on the liquid during purification by flotation
A)a waste material that is the product of smelting a metal ore
B)the aqueous portion of the gangue separated from a metal ore
C)a low-density waste product, formed from the reaction of gangue with another substance, that can be easily separated from the metal ore of interest
D)a substance that reacts with the gangue to make a low-density liquid compound that can be easily separated from the metal of interest
E)a low-density material that floats on the liquid during purification by flotation

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is TRUE of powder metallurgy?
A)Machining is required after forming the component.
B)There is more waste than with casting the molten metal.
C)Iron powder from mill scrap makes a denser component than iron particles formed through water atomization.
D)It is used to separate gangue from the rest of the metal ore.
E)It usually requires lower temperatures than casting the molten metal.
A)Machining is required after forming the component.
B)There is more waste than with casting the molten metal.
C)Iron powder from mill scrap makes a denser component than iron particles formed through water atomization.
D)It is used to separate gangue from the rest of the metal ore.
E)It usually requires lower temperatures than casting the molten metal.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
20
Hydrometallurgy is
A)refining of metal ores using oxidation-reduction reactions.
B)refining of metal ores using heat.
C)refining of metal ores using reactions with aqueous solutions.
D)forming metal parts using heat and small crystals of metal.
E)forming tiny metal crystals using heat and water spray.
A)refining of metal ores using oxidation-reduction reactions.
B)refining of metal ores using heat.
C)refining of metal ores using reactions with aqueous solutions.
D)forming metal parts using heat and small crystals of metal.
E)forming tiny metal crystals using heat and water spray.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
21
Match the following.
Pb
A)sphalerite
B)rhodochrosite
C)rutile
D)carnotite
E)malachite
F)galena
G)cinnabar
Pb
A)sphalerite
B)rhodochrosite
C)rutile
D)carnotite
E)malachite
F)galena
G)cinnabar

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
22
Match the following.
V
A)sphalerite
B)rhodochrosite
C)rutile
D)carnotite
E)malachite
F)galena
G)cinnabar
V
A)sphalerite
B)rhodochrosite
C)rutile
D)carnotite
E)malachite
F)galena
G)cinnabar

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
23
An interstitial alloy contains carbon in one-fourth of the octahedral holes of a closest-packed metal, M. What is the formula of this alloy?
A)M4C
B)MC4
C)MC
D)M2C
E)MC2
A)M4C
B)MC4
C)MC
D)M2C
E)MC2

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
24
Which substance is the best conductor of electricity?
A)arsenic
B)boron
C)silver
D)tellurium
A)arsenic
B)boron
C)silver
D)tellurium

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is TRUE concerning substitutional alloys?
A)The metals involved must have similar radii.
B)One type of metal atom is inserted in between the metal atoms in the initial crystal structure of the other metal.
C)The two metals must be miscible over the entire composition range.
D)Titanium carbide is an example of a substitutional alloy.
E)The metals forming a substitutional alloy must have very different electronegativities.
A)The metals involved must have similar radii.
B)One type of metal atom is inserted in between the metal atoms in the initial crystal structure of the other metal.
C)The two metals must be miscible over the entire composition range.
D)Titanium carbide is an example of a substitutional alloy.
E)The metals forming a substitutional alloy must have very different electronegativities.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is an example of a hydrometallurgical process?
A)Al(OH)3(s)+ SiO2(s)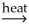 AlSiO3(s)+ 3H2O(g)
AlSiO3(s)+ 3H2O(g)
B)Ni(OH)2(s)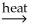 NiO(s)+ H2O(g)
NiO(s)+ H2O(g)
C)2Al(OH)3(s)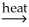 Al2O3(s)+ 3H2O(g)
Al2O3(s)+ 3H2O(g)
D)CuSO4∙5H2O(s)+ H2O(l)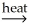 CuS(s)+ H2SO4(aq)+ 5OH⁻(aq)
CuS(s)+ H2SO4(aq)+ 5OH⁻(aq)
E)Al2O3∙nH2O(s)+ 2OH⁻(aq)+ 2H2O(l)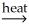 2Al(OH)4⁻(aq)
2Al(OH)4⁻(aq)
A)Al(OH)3(s)+ SiO2(s)
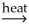 AlSiO3(s)+ 3H2O(g)
AlSiO3(s)+ 3H2O(g)B)Ni(OH)2(s)
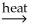 NiO(s)+ H2O(g)
NiO(s)+ H2O(g)C)2Al(OH)3(s)
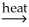 Al2O3(s)+ 3H2O(g)
Al2O3(s)+ 3H2O(g)D)CuSO4∙5H2O(s)+ H2O(l)
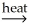 CuS(s)+ H2SO4(aq)+ 5OH⁻(aq)
CuS(s)+ H2SO4(aq)+ 5OH⁻(aq)E)Al2O3∙nH2O(s)+ 2OH⁻(aq)+ 2H2O(l)
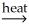 2Al(OH)4⁻(aq)
2Al(OH)4⁻(aq)
Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
27
Identify a balanced equation for the calcination of NiCO3.
A)NiCO3(s)+ CaO(s)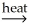 NiO(s)+ CaCO3(s)
NiO(s)+ CaCO3(s)
B) NiCO3(s)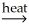 NiO(s)+ CO2(g)
NiO(s)+ CO2(g)
C)NiCO3(s)+ C(s)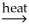 NiO(s)+ 2CO(g)
NiO(s)+ 2CO(g)
D)NiCO3(s)+ SiO2(s)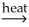 NiSiO3(s)+ CO2(g)
NiSiO3(s)+ CO2(g)
E)NiCO3(s)+ CO(g) NiO(s)+ 2CO2(g)
NiO(s)+ 2CO2(g)
A)NiCO3(s)+ CaO(s)
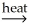 NiO(s)+ CaCO3(s)
NiO(s)+ CaCO3(s)B) NiCO3(s)
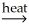 NiO(s)+ CO2(g)
NiO(s)+ CO2(g)C)NiCO3(s)+ C(s)
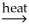 NiO(s)+ 2CO(g)
NiO(s)+ 2CO(g)D)NiCO3(s)+ SiO2(s)
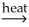 NiSiO3(s)+ CO2(g)
NiSiO3(s)+ CO2(g)E)NiCO3(s)+ CO(g)
 NiO(s)+ 2CO2(g)
NiO(s)+ 2CO2(g)
Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
28
What is the highest oxidation number of chromium?
A)+3
B)+4
C)+5
D)+7
E)+6
A)+3
B)+4
C)+5
D)+7
E)+6

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
29
Identify a balanced reaction for the smelting of SnO2 with carbon.
A)SnO2(s)+ CO2(g)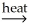 SnCO3(s)
SnCO3(s)
B)SnO2(s)+ CaCO3(s)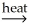 SnC(s)+ CaO(s)+ O2(g)
SnC(s)+ CaO(s)+ O2(g)
C)SnO2(s)+ 2C(s)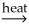 Sn(l)+ 2CO(g)
Sn(l)+ 2CO(g)
D)2SnO2(s)+ 2CaC2(s)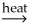 2SnC2(s)+ 2CaO(s)+ O2(g)
2SnC2(s)+ 2CaO(s)+ O2(g)
E)SnO2(s)+ 2CO(g)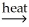 Sn(l)+ 2CO2(g)
Sn(l)+ 2CO2(g)
A)SnO2(s)+ CO2(g)
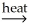 SnCO3(s)
SnCO3(s)B)SnO2(s)+ CaCO3(s)
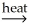 SnC(s)+ CaO(s)+ O2(g)
SnC(s)+ CaO(s)+ O2(g)C)SnO2(s)+ 2C(s)
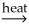 Sn(l)+ 2CO(g)
Sn(l)+ 2CO(g)D)2SnO2(s)+ 2CaC2(s)
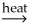 2SnC2(s)+ 2CaO(s)+ O2(g)
2SnC2(s)+ 2CaO(s)+ O2(g)E)SnO2(s)+ 2CO(g)
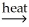 Sn(l)+ 2CO2(g)
Sn(l)+ 2CO2(g)
Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
30
Identify a balanced equation for the calcination of Al(OH)3.
A)2Al(OH)3(s)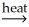 Al2O3(s)+ 3H2O(g)
Al2O3(s)+ 3H2O(g)
B)Al(OH)3(s)+ SiO2(s)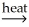 AlSiO3(s)+ 3H2O(g)
AlSiO3(s)+ 3H2O(g)
C)2Al(OH)3(s)+ O2(g)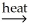 Al2O3(s)+ 3H2O(g)
Al2O3(s)+ 3H2O(g)
D)Al(OH)3(s)+ C(s)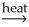 Al2O3(s)+ CO2(g)+ H2O(g)
Al2O3(s)+ CO2(g)+ H2O(g)
E)2Al(OH)3(s)+ 3CO(g)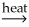 Al2O3(s)+ 3CO2(g)+ 3H2O(g)
Al2O3(s)+ 3CO2(g)+ 3H2O(g)
A)2Al(OH)3(s)
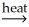 Al2O3(s)+ 3H2O(g)
Al2O3(s)+ 3H2O(g)B)Al(OH)3(s)+ SiO2(s)
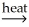 AlSiO3(s)+ 3H2O(g)
AlSiO3(s)+ 3H2O(g)C)2Al(OH)3(s)+ O2(g)
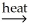 Al2O3(s)+ 3H2O(g)
Al2O3(s)+ 3H2O(g)D)Al(OH)3(s)+ C(s)
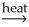 Al2O3(s)+ CO2(g)+ H2O(g)
Al2O3(s)+ CO2(g)+ H2O(g)E)2Al(OH)3(s)+ 3CO(g)
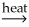 Al2O3(s)+ 3CO2(g)+ 3H2O(g)
Al2O3(s)+ 3CO2(g)+ 3H2O(g)
Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
31
Why can't chromium and nickel form a miscible solid solution over the entire composition range?
A)The lever rule says that these two metals can't coexist in an alloy.
B)The two metals crystallize into different cubic structures in their pure forms.
C)The tetrahedral holes in the nickel crystal are not large enough to accommodate the chromium.
D)The octahedral holes in the chromium crystal are not large enough to accommodate the nickel.
E)Chromium atoms can only fit inside tetrahedral holes, which are not present in the structure of pure nickel.
A)The lever rule says that these two metals can't coexist in an alloy.
B)The two metals crystallize into different cubic structures in their pure forms.
C)The tetrahedral holes in the nickel crystal are not large enough to accommodate the chromium.
D)The octahedral holes in the chromium crystal are not large enough to accommodate the nickel.
E)Chromium atoms can only fit inside tetrahedral holes, which are not present in the structure of pure nickel.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
32
What are the components of bronze?
A)copper and tin
B)lead and aluminum
C)nickel and zinc
D)copper and zinc
E)zinc and lead
A)copper and tin
B)lead and aluminum
C)nickel and zinc
D)copper and zinc
E)zinc and lead

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
33
Match the following.
Hg
A)sphalerite
B)rhodochrosite
C)rutile
D)carnotite
E)malachite
F)galena
G)cinnabar
Hg
A)sphalerite
B)rhodochrosite
C)rutile
D)carnotite
E)malachite
F)galena
G)cinnabar

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
34
What are the components of brass?
A)copper and tin
B)lead and aluminum
C)nickel and zinc
D)copper and zinc
E)zinc and lead
A)copper and tin
B)lead and aluminum
C)nickel and zinc
D)copper and zinc
E)zinc and lead

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
35
An interstitial alloy contains hydrogen in half of the tetrahedral holes of a closest-packed metal, M. What is the formula of this alloy?
A)MH
B)MH4
C)MH2
D)M2H
E)M4H
A)MH
B)MH4
C)MH2
D)M2H
E)M4H

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is TRUE concerning interstitial alloys?
A)The metal takes on the properties of the foreign atoms once they are present.
B)Small atoms fit in between the metal atoms in the crystal structure.
C)Brass is an example of an interstitial alloy.
D)The foreign atoms present weaken the metallic structure.
E)They are alloys composed of two nonmetallic elements.
A)The metal takes on the properties of the foreign atoms once they are present.
B)Small atoms fit in between the metal atoms in the crystal structure.
C)Brass is an example of an interstitial alloy.
D)The foreign atoms present weaken the metallic structure.
E)They are alloys composed of two nonmetallic elements.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is TRUE concerning point "B" on the figure? 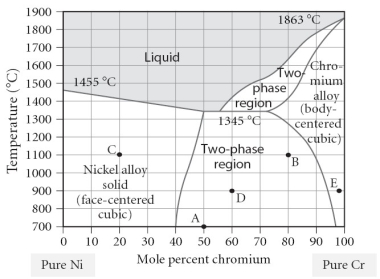
A)The alloy is composed of the BCC structure.
B)The alloy is composed of the FCC structure.
C)The alloy is composed of both FCC and BCC phases, with a larger portion of BCC present.
D)The alloy is composed of both FCC and BCC phases, with a larger portion of FCC present.
E)The alloy is composed of both FCC and BCC phases, with equal portions of each phase.

A)The alloy is composed of the BCC structure.
B)The alloy is composed of the FCC structure.
C)The alloy is composed of both FCC and BCC phases, with a larger portion of BCC present.
D)The alloy is composed of both FCC and BCC phases, with a larger portion of FCC present.
E)The alloy is composed of both FCC and BCC phases, with equal portions of each phase.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
38
Identify the normal crystal structure at room temperature for Ni.
A)face-centred cubic
B)body-centred cubic
C)tetrahedral
D)hexagonal
E)hexagonal closest packed
A)face-centred cubic
B)body-centred cubic
C)tetrahedral
D)hexagonal
E)hexagonal closest packed

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
39
An interstitial alloy contains nitrogen in half of the octahedral holes of a closest-packed metal, M. What is the formula of this alloy?
A)MN
B)MN2
C)M4N
D)M2N
E)MN4
A)MN
B)MN2
C)M4N
D)M2N
E)MN4

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is TRUE concerning point "D" on the figure? 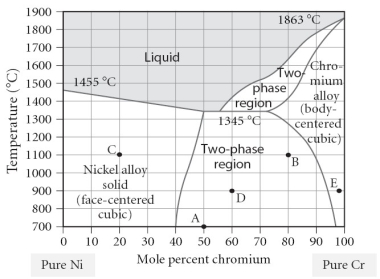
A)The alloy is composed of the BCC structure.
B)The alloy is composed of the FCC structure.
C)The alloy is composed of both FCC and BCC phases, with a larger portion of FCC present.
D)The alloy is composed of both FCC and BCC phases, with a larger portion of BCC present.
E)The alloy is composed of both FCC and BCC phases, with equal portions of each phase.

A)The alloy is composed of the BCC structure.
B)The alloy is composed of the FCC structure.
C)The alloy is composed of both FCC and BCC phases, with a larger portion of FCC present.
D)The alloy is composed of both FCC and BCC phases, with a larger portion of BCC present.
E)The alloy is composed of both FCC and BCC phases, with equal portions of each phase.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
41
Match the following.
Mn
A)sphalerite
B)rhodochrosite
C)rutile
D)carnotite
E)malachite
F)galena
G)cinnabar
Mn
A)sphalerite
B)rhodochrosite
C)rutile
D)carnotite
E)malachite
F)galena
G)cinnabar

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
42
Match the following.
Ti
A)sphalerite
B)rhodochrosite
C)rutile
D)carnotite
E)malachite
F)galena
G)cinnabar
Ti
A)sphalerite
B)rhodochrosite
C)rutile
D)carnotite
E)malachite
F)galena
G)cinnabar

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
43
Match the following.
Zn
A)sphalerite
B)rhodochrosite
C)rutile
D)carnotite
E)malachite
F)galena
G)cinnabar
Zn
A)sphalerite
B)rhodochrosite
C)rutile
D)carnotite
E)malachite
F)galena
G)cinnabar

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
44
Why is zinc used to coat steel objects?

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
45
What is an advantage of hydrometallurgical processes over pyrometallurgical processes?

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
46
Why does a "two-phase" structure occur in a substitutional alloy?

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
47
Describe the difference between a substitutional alloy and an interstitial alloy.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
48
Match the following.
Cu
A)sphalerite
B)rhodochrosite
C)rutile
D)carnotite
E)malachite
F)galena
G)cinnabar
Cu
A)sphalerite
B)rhodochrosite
C)rutile
D)carnotite
E)malachite
F)galena
G)cinnabar

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
49
What is the difference between ferromagnetism and paramagnetism?

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck



