Deck 12: Health Medicine
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/47
Play
Full screen (f)
Deck 12: Health Medicine
1
Which is not true concerning the discovery and development of the penicillin antibiotics?
A)Alexander Fleming noted that the mold Penicillium notatum inhibited bacterial growth
B)The first step in the development of these drugs was the isolation of the active agent produced by the mold Penicillium notatum
C)There are currently more than a dozen different penicillins in clinical use
D)World War I gave added impetus for the development of the penicillin antibiotics
A)Alexander Fleming noted that the mold Penicillium notatum inhibited bacterial growth
B)The first step in the development of these drugs was the isolation of the active agent produced by the mold Penicillium notatum
C)There are currently more than a dozen different penicillins in clinical use
D)World War I gave added impetus for the development of the penicillin antibiotics
World War I gave added impetus for the development of the penicillin antibiotics
2
Isopentyl acetate (shown here) is used as a flavoring agent in food.Its fragrance is that of bananas.What functional group(s) is(are) present in this compound? 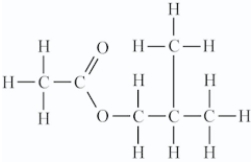
A)Ester only
B)Ketone only
C)Ketone and ether
D)Ester,ether,and ketone

A)Ester only
B)Ketone only
C)Ketone and ether
D)Ester,ether,and ketone
Ester only
3
Which compound cannot exist as a pair of optical isomers?
A)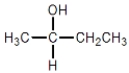
B)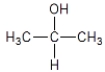
C)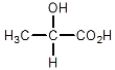
D)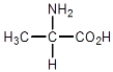
A)

B)

C)

D)


4
Enzymes are
A)biochemical catalysts that influence the rate of chemical reactions.
B)compounds used to relieve inflammation in muscles and joints.
C)proteins that produce fever and swelling.
D)special hormones that influence the rate of chemical reactions.
A)biochemical catalysts that influence the rate of chemical reactions.
B)compounds used to relieve inflammation in muscles and joints.
C)proteins that produce fever and swelling.
D)special hormones that influence the rate of chemical reactions.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
5
Isomers are compounds that have
A)the same chemical formulas and molecular structures but different physical properties.
B)the same chemical formulas but different molecular structures and physical properties.
C)different chemical formulas and molecular structures but the same physical properties.
D)different chemical formulas,molecular structures,and physical properties.
A)the same chemical formulas and molecular structures but different physical properties.
B)the same chemical formulas but different molecular structures and physical properties.
C)different chemical formulas and molecular structures but the same physical properties.
D)different chemical formulas,molecular structures,and physical properties.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
6
Identify the chiral carbon atom in L-dopa (used to treat Parkinson's disease). 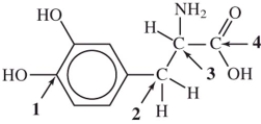
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
7
What is the structure of the ester that is formed in the following reaction? 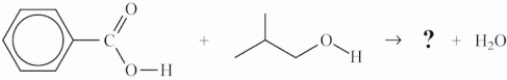
A)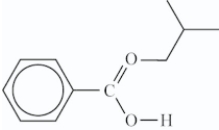
B)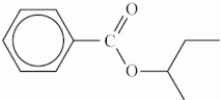
C)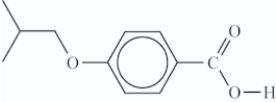
D)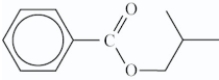

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
8
The father of modern medicine,Hippocrates,described making a tea that was effective against fever by boiling willow bark.Years later,the active ingredient was isolated and chemically modified to produce the drug we now know as
A)acetaminophen.
B)aspirin.
C)ibuprofen.
D)penicillin.
A)acetaminophen.
B)aspirin.
C)ibuprofen.
D)penicillin.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
9
The common OTC medication ibuprofen is sold as a racemic mixture although only the L-isomer is a pain reliever.What does the term racemic mixture mean?
A)There is an equal mixture of the + and - optical isomers in the drug preparation
B)There are two active ingredients in the drug preparation
C)The inactive optical isomer does not cause serious side effects
D)The inactive isomer is transformed into the active isomer in the body
A)There is an equal mixture of the + and - optical isomers in the drug preparation
B)There are two active ingredients in the drug preparation
C)The inactive optical isomer does not cause serious side effects
D)The inactive isomer is transformed into the active isomer in the body

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
10
Which of these incorrectly pairs a hormone with its function?
A)Thyroxin-regulates metabolism
B)Insulin-enables the body to use glucose (blood sugar) for energy
C)Adrenaline (epinephrine)-prepares the body to "fight or flee"
D)Progesterone-controls muscle mass
A)Thyroxin-regulates metabolism
B)Insulin-enables the body to use glucose (blood sugar) for energy
C)Adrenaline (epinephrine)-prepares the body to "fight or flee"
D)Progesterone-controls muscle mass

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
11
The body's chemical messengers,called ________,are produced in the endocrine glands.
A)enzymes
B)hormones
C)isomers
D)viruses
A)enzymes
B)hormones
C)isomers
D)viruses

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
12
The octet rule is applied to bonding in carbon containing compounds.In accordance with the octet rule,
A)each bonded carbon atom forms eight bonds.
B)all carbon containing compounds contain at least eight bonds.
C)each bonded carbon atom shares electrons so that it has exactly eight outer valence electrons.
D)the number of atoms in any carbon containing compound is a multiple of eight.
A)each bonded carbon atom forms eight bonds.
B)all carbon containing compounds contain at least eight bonds.
C)each bonded carbon atom shares electrons so that it has exactly eight outer valence electrons.
D)the number of atoms in any carbon containing compound is a multiple of eight.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
13
The term "pharmacophore" was originally described by the Nobel Prize winner Paul Ehrlich more than 100 years ago.What is the meaning of this term?
A)The correct dose at which a drug should be administered
B)The three dimensional arrangement of a drug responsible for its biological activity
C)The form in which a drug is prepared for oral consumption
D)The group of compounds responsible for euphoria
A)The correct dose at which a drug should be administered
B)The three dimensional arrangement of a drug responsible for its biological activity
C)The form in which a drug is prepared for oral consumption
D)The group of compounds responsible for euphoria

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
14
For linear n-hexane,C6H14,which molecular representation is correct?
A)
B)
C)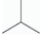
D)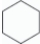
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
15
Amino acids are compounds that contain both amine and organic acid functional groups.Which compound is an amino acid?
A)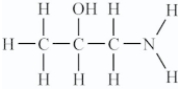
B)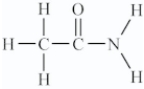
C)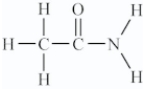
D)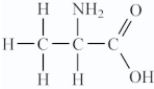
A)

B)
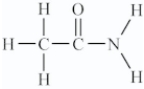
C)
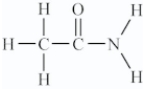
D)
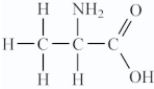

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
16
Which pair represents isomers?
A)
B)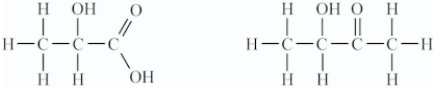
C)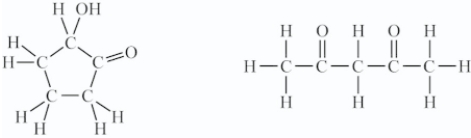
D)
A)

B)
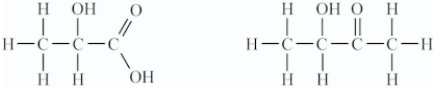
C)

D)


Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
17
Which functional group does this molecule contain? 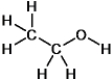
A)Acid
B)Alcohol
C)Amine
D)Ester

A)Acid
B)Alcohol
C)Amine
D)Ester

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
18
For this line-angle drawing,which condensed structural formula is correct? 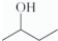
A)CH3CH2CH2CH2OH
B)CH3CH(CH3)CH2OH
C)CH3CH(OH)CH2CH3
D)CH(CH3)2CH2OH

A)CH3CH2CH2CH2OH
B)CH3CH(CH3)CH2OH
C)CH3CH(OH)CH2CH3
D)CH(CH3)2CH2OH

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
19
Which functional group contains a nitrogen atom?
A)Alcohol
B)Amine
C)Ester
D)Ether
A)Alcohol
B)Amine
C)Ester
D)Ether

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
20
Why are many drugs administered as salts rather than as the corresponding neutral compounds?
A)Salts have greater water solubility and stability than the neutral compound
B)As a general rule,salts are more active than the neutral compounds
C)Salts are generally liquid and therefore more active than the neutral compounds
D)The salts are less expensive to produce than the corresponding neutral compounds
A)Salts have greater water solubility and stability than the neutral compound
B)As a general rule,salts are more active than the neutral compounds
C)Salts are generally liquid and therefore more active than the neutral compounds
D)The salts are less expensive to produce than the corresponding neutral compounds

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
21
Which region of cholesterol is most responsible for its solubility in nonpolar substances? 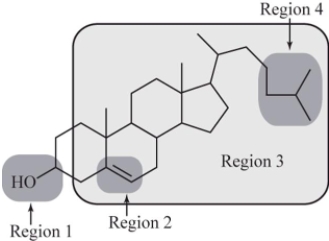
A)Region 1
B)Region 2
C)Region 3
D)Region 4

A)Region 1
B)Region 2
C)Region 3
D)Region 4

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
22
Select the formula for the equilibrium constant for the association of epinephrine and its receptor. epinephrine + receptor à complex
A)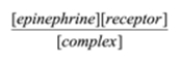
B)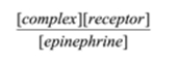
B
C)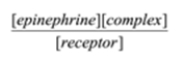
D)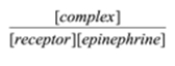
A)
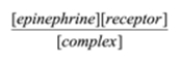
B)
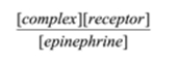
B
C)
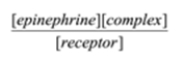
D)
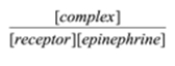

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
23
What functional groups are common to both of structures below? 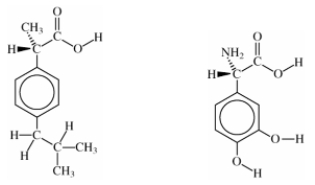
A)Acid
B)Alcohol
C)Amide
D)Amine

A)Acid
B)Alcohol
C)Amide
D)Amine

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
24
High fructose corn syrup is produced from the partial conversion of glucose to fructose.For this reaction K=0.74.If the glucose concentration is 5x10-3 M,the fructose concentration must equal
A)6.8x10-3 M.
B)3.7x10-3 M.
C)4.0x10-2 M.
D)1.4x10-4 M.
A)6.8x10-3 M.
B)3.7x10-3 M.
C)4.0x10-2 M.
D)1.4x10-4 M.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
25
If the reaction quotient,Q,is less than the equilibrium constant,Keq,the reaction will
A)continue both forward and reverse reactions to keep the ratio of reactants and products the same.
B)continue the forward reaction to convert reactants into products.
C)continue the reverse reaction to convert products into reactants.
D)halt all reactions.
A)continue both forward and reverse reactions to keep the ratio of reactants and products the same.
B)continue the forward reaction to convert reactants into products.
C)continue the reverse reaction to convert products into reactants.
D)halt all reactions.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
26
The family of compounds known as steroids share a common molecular framework of 17 carbon atoms that are arranged into four rings.Which is a representation of this common molecular framework?
A)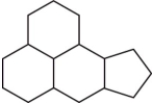
B)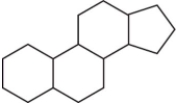
C)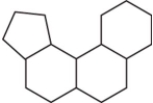
D)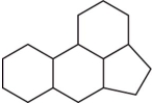
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
27
Some steroids are more soluble in water than others.Based on your understanding of the solubility of molecular compounds in water,decide which compound is the most soluble.
A)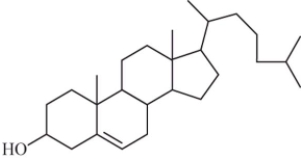
B)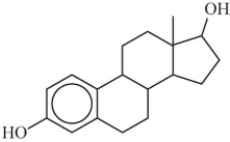
C)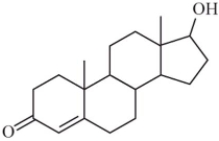
D)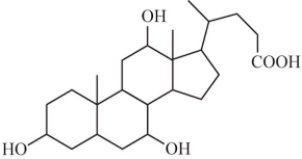
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
28
The equilibrium constant for the association of thyroxin with its receptor is greater than 1.Given the reaction below,which of the following statements are true. thyroxin + receptor à complex
A)Unbound thyroxin will be favored over bound
B)Bound thyroxin will be favored over unbound
C)The receptor has a very low concentration in the body
D)The hormone,thyroxin,has a very low concentration in the body
A)Unbound thyroxin will be favored over bound
B)Bound thyroxin will be favored over unbound
C)The receptor has a very low concentration in the body
D)The hormone,thyroxin,has a very low concentration in the body

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
29
If the reaction quotient,Q,is higher than the equilibrium constant,Keq,the reaction will
A)continue both forward and reverse reactions to keep the ratio of reactants and products the same.
B)continue the forward reaction to convert reactants into products.
C)continue the reverse reaction to convert products into reactants.
D)halt all reactions.
A)continue both forward and reverse reactions to keep the ratio of reactants and products the same.
B)continue the forward reaction to convert reactants into products.
C)continue the reverse reaction to convert products into reactants.
D)halt all reactions.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following are general goals for green chemical synthesis methods? I.There is less waste
II)Harsh reagents are avoided
III)It uses recombinant technology
IV)It needs intense purification
A)I and IV only
B)II and III only
C)III and IV only
D)I,II,and III only
II)Harsh reagents are avoided
III)It uses recombinant technology
IV)It needs intense purification
A)I and IV only
B)II and III only
C)III and IV only
D)I,II,and III only

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
31
Acetic acid has a pKa of 4.8.Select the representation that shows the majority species at pH 7.
A)CH3COOH2+
B)CH3COOH
C)CH3COO-
D)CH2COO2-
A)CH3COOH2+
B)CH3COOH
C)CH3COO-
D)CH2COO2-

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
32
What structural feature do the two hormones shown have in common? 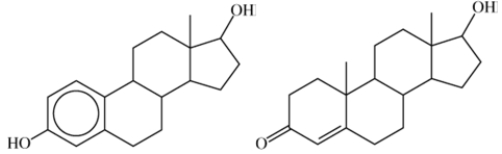
A)Both have alcohol functional groups
B)Both have ketone functional groups
C)Both are steroids
D)There are no structural features in common

A)Both have alcohol functional groups
B)Both have ketone functional groups
C)Both are steroids
D)There are no structural features in common

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
33
Ammonium (NH4+) has a pKa of 9.3.Select the majority species at pH = 10
A)NH3+
B)NH3.
C)NH4+
D)NH5+
A)NH3+
B)NH3.
C)NH4+
D)NH5+

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
34
Comparing the structures of aspirin,ibuprofen,and acetaminophen,what structural units do they share? 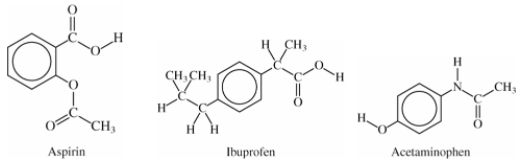
A)Acid functional groups
B)Amide functional groups
C)Benzene rings
D)Ester functional groups

A)Acid functional groups
B)Amide functional groups
C)Benzene rings
D)Ester functional groups

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
35
Select the true statement about conjugate bases.
A)A conjugate base is one product of the reaction of a carboxylic acid with an ester
B)Conjugate bases are only important for buffers made with strong acids
C)A conjugate base is one dissociation product of any acid
D)None of these choices are true about conjugate bases
A)A conjugate base is one product of the reaction of a carboxylic acid with an ester
B)Conjugate bases are only important for buffers made with strong acids
C)A conjugate base is one dissociation product of any acid
D)None of these choices are true about conjugate bases

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
36
Select the correct statement regarding weak and strong acids.
A)A weak acid will resist changes to pH
B)A strong acid will resist changes to pH
C)A strong acid will not release its protons easily
D)A weak acid will completely dissociate in water
A)A weak acid will resist changes to pH
B)A strong acid will resist changes to pH
C)A strong acid will not release its protons easily
D)A weak acid will completely dissociate in water

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
37
Only three of the statements about buffers in the body below are correct.Select the false statement.
A)Organs or cell components will differ in their normal pH
B)Buffers are only necessary in the blood and stomach
C)Weak acids only make good buffers when the pH is near their pKa
D)Strong acids will not make good buffers
A)Organs or cell components will differ in their normal pH
B)Buffers are only necessary in the blood and stomach
C)Weak acids only make good buffers when the pH is near their pKa
D)Strong acids will not make good buffers

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
38
If the reaction quotient,Q,is equal to the equilibrium constant,Keq,the reaction will
A)continue both forward and reverse reactions to keep the ratio of reactants and products the same.
B)continue the forward reaction to convert reactants into products.
C)continue the reverse reaction to convert products into reactants.
D)halt all reactions.
A)continue both forward and reverse reactions to keep the ratio of reactants and products the same.
B)continue the forward reaction to convert reactants into products.
C)continue the reverse reaction to convert products into reactants.
D)halt all reactions.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
39
Formic acid has a pKa of 3.8.Calculate the pH of a solution if [Formic acid] = 0.020 M and [Formate] = 0.040 M.
A)pH = 3.5
B)pH = 3.8
C)pH = 4.1
D)pH = 4.4
A)pH = 3.5
B)pH = 3.8
C)pH = 4.1
D)pH = 4.4

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
40
Identify groups of structural isomers among the compounds below. 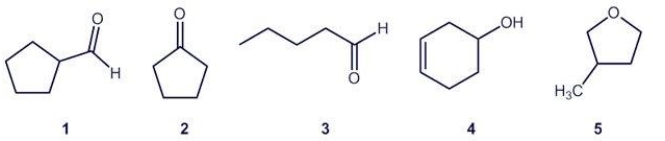
A)3 and 5 AND 1 and 4
B)2 and 3
C)1,3 and 5
D)1 and 4

A)3 and 5 AND 1 and 4
B)2 and 3
C)1,3 and 5
D)1 and 4

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
41
Vitamin E has many promising pharmacological benefits.Which of the following statements about vitamin E are false?
A)Vitamin E is a mixture of structural and optical isomers
B)Biological systems will only absorb and concentrate some vitamin E isomers
C)Vitamin E plays a role in fetal development
D)The L-form of vitamin E can cause accelerated heart rate
A)Vitamin E is a mixture of structural and optical isomers
B)Biological systems will only absorb and concentrate some vitamin E isomers
C)Vitamin E plays a role in fetal development
D)The L-form of vitamin E can cause accelerated heart rate

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
42
Radioisotopes and ionizing radiation I.allow us to develop high resolution images of some tissues.
II)were hypothesized for medical use over a century ago.
III)can induce apoptosis in cancerous tissues.
IV)are always selective for cancerous tissues.
A)I and III
B)II and IV
C)I,II,and III
D)I,II,III,and IV
II)were hypothesized for medical use over a century ago.
III)can induce apoptosis in cancerous tissues.
IV)are always selective for cancerous tissues.
A)I and III
B)II and IV
C)I,II,and III
D)I,II,III,and IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
43
Glycolysis I.is stage one of cellular respiration.
II)converts glucose to smaller high energy compounds.
III)requires oxygen to operate.
IV)is utilized by muscles for immediate energy.
A)I and III
B)II,III,and IV
C)I,II,and IV
D)I,II,III,and IV
II)converts glucose to smaller high energy compounds.
III)requires oxygen to operate.
IV)is utilized by muscles for immediate energy.
A)I and III
B)II,III,and IV
C)I,II,and IV
D)I,II,III,and IV

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is not a correct pair a biological macromolecule class with one of its functions?
A)Nucleic acids;information transfer
B)Lipids;protection
C)Proteins;cellular structure
D)Polysaccharides;catalysis
A)Nucleic acids;information transfer
B)Lipids;protection
C)Proteins;cellular structure
D)Polysaccharides;catalysis

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
45
Modern drug discovery often starts with a large library of compounds.These library studies are important because
A)cancerous tissue is much more difficult to target than foreign invaders.
B)old drugs will never be effective against new targets.
C)the search will definitely yield a new candidate drug.
D)the search may yield a number of possible framework pieces to build into a good drug.
A)cancerous tissue is much more difficult to target than foreign invaders.
B)old drugs will never be effective against new targets.
C)the search will definitely yield a new candidate drug.
D)the search may yield a number of possible framework pieces to build into a good drug.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
46
A structure-activity-relationship study
A)considers the relationship of many enzymes based upon both their structure and activity.
B)examines the structure of an enzyme then compares it to its activity with the drug.
C)makes a series of small structural changes to the drug then compares it to its activity with the enzyme.
D)isolates small portions of the protein to examine how their structure relates to their activity.
A)considers the relationship of many enzymes based upon both their structure and activity.
B)examines the structure of an enzyme then compares it to its activity with the drug.
C)makes a series of small structural changes to the drug then compares it to its activity with the enzyme.
D)isolates small portions of the protein to examine how their structure relates to their activity.

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is not a class of biological macromolecule?
A)Steroids
B)Lipids
C)Nucleic acids
D)Polysaccharides
A)Steroids
B)Lipids
C)Nucleic acids
D)Polysaccharides

Unlock Deck
Unlock for access to all 47 flashcards in this deck.
Unlock Deck
k this deck



