Deck 7: Energy Storage
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/18
Play
Full screen (f)
Deck 7: Energy Storage
1
What are the typical units for specific energy?
A)mAh/g
B)W/kg
C)Wh/kg
D)kWh
A)mAh/g
B)W/kg
C)Wh/kg
D)kWh
Wh/kg
2
How do the interactions that are broken in water when it is boiled compare with those broken when water is electrolyzed?
A)Boiling water breaks intermolecular attractions and electrolysis breaks covalent bonds
B)Boiling water breaks covalent bonds and electrolysis breaks intermolecular attractions
C)Boiling water and electrolysis of water break covalent bonds
D)Boiling water and electrolysis of water break intermolecular forces
A)Boiling water breaks intermolecular attractions and electrolysis breaks covalent bonds
B)Boiling water breaks covalent bonds and electrolysis breaks intermolecular attractions
C)Boiling water and electrolysis of water break covalent bonds
D)Boiling water and electrolysis of water break intermolecular forces
Boiling water breaks intermolecular attractions and electrolysis breaks covalent bonds
3
For the compound Fe3O4,what is the oxidation state of Fe?
A)2+
B)3+
C)4+
D)Mixture between 2+ and 3+
A)2+
B)3+
C)4+
D)Mixture between 2+ and 3+
Mixture between 2+ and 3+
4
A NiCd battery uses nickel and cadmium to produce a potential difference.Using these equations,answer the following questions.
I.2NiO(OH) (s) + 2H2O (l) + 2 e¯ 2Ni(OH)2 (s) + 2 OH¯ (aq)
II.Cd(s) + 2OH¯ (aq) Cd(OH)2 (s) + 2e¯
III.Cd (s) + 2NiO(OH) (s) + 2 H2O (l) 2 Ni(OH)2 (s) + Cd(OH)2 (s)
Which equation represents what takes place at the anode?
A)I
B)II
C)III
D)I and II
I.2NiO(OH) (s) + 2H2O (l) + 2 e¯ 2Ni(OH)2 (s) + 2 OH¯ (aq)
II.Cd(s) + 2OH¯ (aq) Cd(OH)2 (s) + 2e¯
III.Cd (s) + 2NiO(OH) (s) + 2 H2O (l) 2 Ni(OH)2 (s) + Cd(OH)2 (s)
Which equation represents what takes place at the anode?
A)I
B)II
C)III
D)I and II

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
5
In this electrochemical cell,the reduction half reaction is 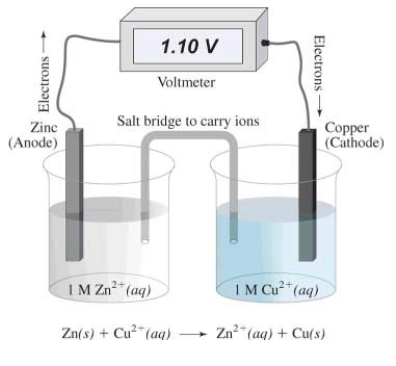
A)Cu2+(aq) + 2 e¯ Cu(s)
B)Zn(s) Zn2+(aq) + 2 e¯
C)Zn(s) Cu(s)
D)Cu2+(aq) Zn2+(aq)

A)Cu2+(aq) + 2 e¯ Cu(s)
B)Zn(s) Zn2+(aq) + 2 e¯
C)Zn(s) Cu(s)
D)Cu2+(aq) Zn2+(aq)

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
6
During the chemical reaction in an electrochemical cell,
A)a substance is oxidized and gains electrons.
B)electrons travel from the cathode to the anode.
C)oxidation takes place alone,without an accompanying reduction.
D)oxidation occurs at the anode.
A)a substance is oxidized and gains electrons.
B)electrons travel from the cathode to the anode.
C)oxidation takes place alone,without an accompanying reduction.
D)oxidation occurs at the anode.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements is not true about PEM fuel cells?
A)PEM fuel cells rely on inexpensive catalysts
B)PEM fuels cells are cleaner than gasoline powered vehicles
C)PEM fuel cells have safe and efficient fuel storage
D)PEM fuel cells have limited driving range
A)PEM fuel cells rely on inexpensive catalysts
B)PEM fuels cells are cleaner than gasoline powered vehicles
C)PEM fuel cells have safe and efficient fuel storage
D)PEM fuel cells have limited driving range

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
8
Whenever a substance is oxidized,
A)it is called the oxidizing agent.
B)some other substance must be reduced.
C)it gains electrons.
D)hydronium ions are produced.
A)it is called the oxidizing agent.
B)some other substance must be reduced.
C)it gains electrons.
D)hydronium ions are produced.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
9
Why doesn't water naturally become hydrogen and oxygen gas when exposed to light?
A)Thermal energy is the only appropriate type of energy to split water
B)You must use electrons to split water
C)Water doesn't absorb the proper wavelength of light to split
A)Thermal energy is the only appropriate type of energy to split water
B)You must use electrons to split water
C)Water doesn't absorb the proper wavelength of light to split

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
10
In general,a modern hybrid vehicle is less polluting than a standard vehicle because it runs on both a
A)gasoline engine and an electric motor run by a rechargeable battery.
B)gasoline engine and a fuel cell.
C)fuel cell and an electric motor run by a rechargeable battery.
D)gasoline engine and a cleaner diesel engine.
A)gasoline engine and an electric motor run by a rechargeable battery.
B)gasoline engine and a fuel cell.
C)fuel cell and an electric motor run by a rechargeable battery.
D)gasoline engine and a cleaner diesel engine.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
11
Reforming processes can store hydrogen fuel in a liquid form as another molecule.Which of the following cannot be reformed into hydrogen?
A)Methanol
B)Gasoline
C)Diesel
D)Carbon dioxide
A)Methanol
B)Gasoline
C)Diesel
D)Carbon dioxide

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
12
For the compound FeCl3,what is the oxidation state of Fe?
A)2+
B)3+
C)4+
D)Mixture between 2+ and 3+
A)2+
B)3+
C)4+
D)Mixture between 2+ and 3+

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
13
Chemical energy is converted directly into electrical energy in
A)a galvanic cell.
B)an electrical power plant.
C)an electrolytic cell.
D)an automobile's engine.
A)a galvanic cell.
B)an electrical power plant.
C)an electrolytic cell.
D)an automobile's engine.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
14
In a fuel cell,
A)there is oxidation,but no reduction.
B)reduction takes place at the cathode.
C)there is direct conversion of mechanical energy into electricity.
D)a chemical reaction produces heat,which then produces electricity.
A)there is oxidation,but no reduction.
B)reduction takes place at the cathode.
C)there is direct conversion of mechanical energy into electricity.
D)a chemical reaction produces heat,which then produces electricity.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
15
Batteries are still preferred over supercapacitors for portable electronic applications.What factor(s) give rise to this preference?
A)Supercapacitors have low energy densities
B)Supercapacitors are charged much more quickly than batteries
C)Supercapacitors have virtually unlimited cycle lives
D)All of these choices are correct
A)Supercapacitors have low energy densities
B)Supercapacitors are charged much more quickly than batteries
C)Supercapacitors have virtually unlimited cycle lives
D)All of these choices are correct

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
16
Why is it so expensive to ship hydrogen as a liquid as is often done with other gas?
A)Hydrogen is very heavy to ship
B)Hydrogen is very dense to ship
C)Hydrogen has to be shipped at a very low temperature
D)Hydrogen in very flammable
A)Hydrogen is very heavy to ship
B)Hydrogen is very dense to ship
C)Hydrogen has to be shipped at a very low temperature
D)Hydrogen in very flammable

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following automotive energy storage devices would give rise to the fastest acceleration from zero to 60 mph?
A)Li-ion rechargeable batteries
B)Fuel cells
C)NiMH rechargeable batteries
D)Supercapacitors
A)Li-ion rechargeable batteries
B)Fuel cells
C)NiMH rechargeable batteries
D)Supercapacitors

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
18
How does the reaction of hydrogen and oxygen to produce energy in a fuel cell differ from their interaction during the direct combustion of hydrogen and oxygen? 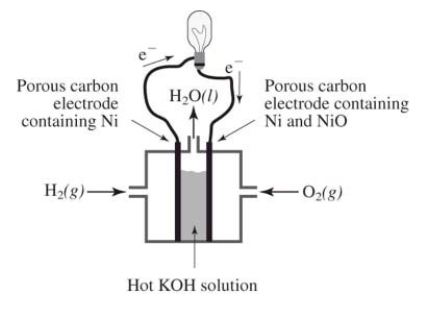 I.The direct combustion of hydrogen and oxygen produces several different products,whereas the fuel cell produces only water
I.The direct combustion of hydrogen and oxygen produces several different products,whereas the fuel cell produces only water
II)Much less heat energy is produced in a fuel cell than via direct combustion of hydrogen and oxygen
III)In the fuel cell,there is an oxidation-reduction reaction between hydrogen and oxygen.In the direct combustion of hydrogen and oxygen,there is no such reaction
IV)It is much easier to control the hydrogen and oxygen during direct combustion than during their reaction in a fuel cell
A)I and II only
B)I,II,and III only
C)I,II,III,and IV
D)I and III only
 I.The direct combustion of hydrogen and oxygen produces several different products,whereas the fuel cell produces only water
I.The direct combustion of hydrogen and oxygen produces several different products,whereas the fuel cell produces only waterII)Much less heat energy is produced in a fuel cell than via direct combustion of hydrogen and oxygen
III)In the fuel cell,there is an oxidation-reduction reaction between hydrogen and oxygen.In the direct combustion of hydrogen and oxygen,there is no such reaction
IV)It is much easier to control the hydrogen and oxygen during direct combustion than during their reaction in a fuel cell
A)I and II only
B)I,II,and III only
C)I,II,III,and IV
D)I and III only

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck



