Deck 5: Energy From Combustion
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/5
Play
Full screen (f)
Deck 5: Energy From Combustion
1
Consider these three compounds. 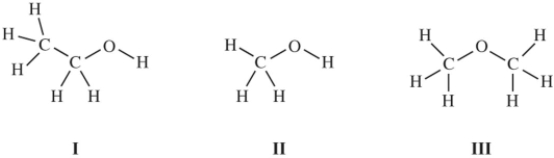 Which are isomers?
Which are isomers?
A)I and II only
B)II and III only
C)I and III only
D)I,II,and III
 Which are isomers?
Which are isomers?A)I and II only
B)II and III only
C)I and III only
D)I,II,and III
I and III only
2
The following molecules contain only single bonds. NH3(g) + 3F2(g) NF3(g) + 3 HF(g)The bond energies are: N - H 391 kJ/mol;F - F 158 kJ/mol;N - F 272 kJ/mol;H - F 566 kJ/mol.Which is the heat evolved or absorbed per mole of NH3 that reacts with F2?
A)+289 kJ/mol
B)+867 kJ/mol
C)-289kJ/mol
D)-867 kJ/mol
A)+289 kJ/mol
B)+867 kJ/mol
C)-289kJ/mol
D)-867 kJ/mol
-867 kJ/mol
3
The heat energy released or absorbed by a chemical reaction is generally determined by the difference between the energy that
A)must be put in to break the bonds in the reactants and the energy that must be put in to make the bonds in the products.
B)must be put in to break the bonds in the reactants and the energy that is released upon making the bonds in the products.
C)is released upon breaking the bonds in the reactants and the energy that must be put in to make the bonds in the products.
D)is released upon breaking the bonds in the reactants and the energy that is released upon making the bonds in the products.
A)must be put in to break the bonds in the reactants and the energy that must be put in to make the bonds in the products.
B)must be put in to break the bonds in the reactants and the energy that is released upon making the bonds in the products.
C)is released upon breaking the bonds in the reactants and the energy that must be put in to make the bonds in the products.
D)is released upon breaking the bonds in the reactants and the energy that is released upon making the bonds in the products.
must be put in to break the bonds in the reactants and the energy that is released upon making the bonds in the products.
4
Consider these three compounds. 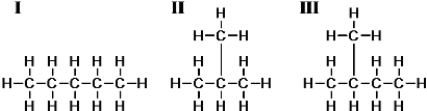 Which are isomers?
Which are isomers?
A)I and II only
B)I and III only
C)II and III only
D)I,II,and III
 Which are isomers?
Which are isomers?A)I and II only
B)I and III only
C)II and III only
D)I,II,and III

Unlock Deck
Unlock for access to all 5 flashcards in this deck.
Unlock Deck
k this deck
5
A calorie is defined as exactly 4.184 J.Therefore 1.000 Cal is exactly
A)41.84 J.
B)418.4 J.
C)1000 J.
D)4184 J.
A)41.84 J.
B)418.4 J.
C)1000 J.
D)4184 J.

Unlock Deck
Unlock for access to all 5 flashcards in this deck.
Unlock Deck
k this deck



