Deck 2: The Air We Breathe
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/50
Play
Full screen (f)
Deck 2: The Air We Breathe
1
Which two gases make up more than 95 percent of an inhaled breath?
A)NO2 and N2
B)CO2 and O2
C)O2 and N2
D)N2 and Ar
A)NO2 and N2
B)CO2 and O2
C)O2 and N2
D)N2 and Ar
O2 and N2
2
The EPA limit for CO is 9 ppm.Express this number as a percentage.
A)90 percent
B)9 percent
C)0.09 percent
D)0.0009 percent
A)90 percent
B)9 percent
C)0.09 percent
D)0.0009 percent
0.0009 percent
3
When assessing the risk of an air pollutant,which does not play a role in considering someone's exposure to the pollutant?
A)A person's lung capacity
B)A person's breathing rate
C)The toxicity of the pollutant
D)The concentration in air of the pollutant
A)A person's lung capacity
B)A person's breathing rate
C)The toxicity of the pollutant
D)The concentration in air of the pollutant
The toxicity of the pollutant
4
Which color,as used in the Air Quality Index,warns that the level of a pollutant is hazardous,the most dangerous level?
A)Orange
B)Green
C)Yellow
D)Maroon
A)Orange
B)Green
C)Yellow
D)Maroon

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
5
Of five major gaseous components of air,which is the only one to vary significantly in concentration from place to place and from day to day?
A)Water vapor
B)Carbon dioxide
C)Nitrogen
D)Argon
A)Water vapor
B)Carbon dioxide
C)Nitrogen
D)Argon

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
6
A particular sample of air is 2.5 percent water vapor.Express the concentration of water vapor in parts per million (ppm).
A)0.0000025 ppm
B)0.025 ppm
C)250 ppm
D)25000 ppm
A)0.0000025 ppm
B)0.025 ppm
C)250 ppm
D)25000 ppm

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
7
Which pollutant are you more likely to encounter in dangerous concentrations indoors rather than outdoors?
A)Nitrogen dioxide
B)Carbon monoxide
C)Ozone
D)Sulfur dioxide
A)Nitrogen dioxide
B)Carbon monoxide
C)Ozone
D)Sulfur dioxide

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
8
What two factors are considered when determining the risk assessment for air pollutants?
A)Exposure and ppm
B)Percentage and ppm
C)Toxicity and percentage
D)Toxicity and exposure
A)Exposure and ppm
B)Percentage and ppm
C)Toxicity and percentage
D)Toxicity and exposure

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
9
Which component of the air is an element?
A)H2O
B)NO2
C)O2
D)CO2
A)H2O
B)NO2
C)O2
D)CO2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
10
The ________ concentration in the air over the desert differs dramatically from that in the air in the tropical rainforest.
A)N2
B)O2
C)CO2
D)H2O
A)N2
B)O2
C)CO2
D)H2O

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
11
Ozone is considered an air pollutant in the ________ but is a valuable protective layer in the ________.
A)troposphere;stratosphere
B)stratosphere;mesosphere
C)stratosphere;troposphere
D)mesosphere;stratosphere
A)troposphere;stratosphere
B)stratosphere;mesosphere
C)stratosphere;troposphere
D)mesosphere;stratosphere

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
12
Air is a(n)
A)element.
B)compound.
C)mixture.
D)pure substance.
A)element.
B)compound.
C)mixture.
D)pure substance.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
13
Which substance is not considered to be an air pollutant?
A)N2
B)SO2
C)NO2
D)O3
A)N2
B)SO2
C)NO2
D)O3

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
14
Based on its name,which carbon compound contains the fewest carbon atoms?
A)Ethanol
B)Methane
C)Chlorobutane
D)Propyl alcohol
A)Ethanol
B)Methane
C)Chlorobutane
D)Propyl alcohol

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
15
In general,which airborne material is not likely to be affected by the filters or indoor air handling equipment?
A)Particulates
B)Pollen
C)Soot
D)Carbon monoxide
A)Particulates
B)Pollen
C)Soot
D)Carbon monoxide

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
16
All of these pollutants can be detected by their odors except
A)CO.
B)O3.
C)SOx.
D)NOx.
A)CO.
B)O3.
C)SOx.
D)NOx.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
17
What is the primary component of an exhaled breath?
A)N2
B)O2
C)CO2
D)H2O
A)N2
B)O2
C)CO2
D)H2O

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
18
Which component of the air makes up approximately 100 times more of an exhaled breath than of an inhaled breath?
A)Ar
B)O2
C)O3
D)CO2
A)Ar
B)O2
C)O3
D)CO2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
19
The burning of coal produces sulfur dioxide,SO2,a pollutant that slowly reacts in air to form SO3.Sulfur trioxide dissolves into airborne water droplets to form a very corrosive solution of sulfuric acid.Which is a product of burning coal that hastens the transformation of sulfur dioxide into sulfur trioxide?
A)Carbon dioxide
B)Carbon monoxide
C)Nitrogen dioxide
D)Particles of ash
A)Carbon dioxide
B)Carbon monoxide
C)Nitrogen dioxide
D)Particles of ash

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
20
Which pollutant is present in air as particulate matter?
A)Soot
B)Ozone
C)Sulfur dioxide
D)Carbon monoxide
A)Soot
B)Ozone
C)Sulfur dioxide
D)Carbon monoxide

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following are examples of technological advances that have reduced air pollution?
A)Paint with reduced VOCs
B)Catalytic converters
C) Burning gasoline in leaf blowers
D)Low sulfur diesel fuels
A)Paint with reduced VOCs
B)Catalytic converters
C) Burning gasoline in leaf blowers
D)Low sulfur diesel fuels

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
22
If 500 mL of air contains 2 x 1022 particles (atoms and molecules),how many particles do you inhale in one day if you breathe 15000 L of air?
A)2 x 1022
B)6 x 1026
C)1.2 x 1027
D)5 x 1024
A)2 x 1022
B)6 x 1026
C)1.2 x 1027
D)5 x 1024

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
23
Ozone is a secondary pollutant.A secondary pollutant is
A)not as hazardous as a primary pollutant.
B)not produced directly but as the product of the interaction of two or more pollutants.
C)one that is naturally present in our atmosphere.
D)one that is less hazardous than a primary pollutant.
A)not as hazardous as a primary pollutant.
B)not produced directly but as the product of the interaction of two or more pollutants.
C)one that is naturally present in our atmosphere.
D)one that is less hazardous than a primary pollutant.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following would be described as "fine particles?"
A)SOx
B)NOx
C)O3
D)2.5 μm diameter soot
A)SOx
B)NOx
C)O3
D)2.5 μm diameter soot

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
25
Which is the balanced chemical equation for the reaction of nitrogen (N2) with oxygen (O2) to form NO?
A)2 NO → N2 + O2
B)N2 + O2 → NO
C)N2 + O2 → 2 NO
D)NO → N2 + O2
A)2 NO → N2 + O2
B)N2 + O2 → NO
C)N2 + O2 → 2 NO
D)NO → N2 + O2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
26
Which shows the balanced equation for the reaction of nitrogen (  ),as it is normally found in our atmosphere,with oxygen (
),as it is normally found in our atmosphere,with oxygen (  ),as it is normally found in our atmosphere,to form nitrogen dioxide?
),as it is normally found in our atmosphere,to form nitrogen dioxide?
A)
B)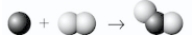
C)
D)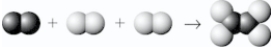
 ),as it is normally found in our atmosphere,with oxygen (
),as it is normally found in our atmosphere,with oxygen (  ),as it is normally found in our atmosphere,to form nitrogen dioxide?
),as it is normally found in our atmosphere,to form nitrogen dioxide?A)

B)

C)

D)


Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
27
Choose the proper coefficients for each substance to balance this equation. ___ C2H4(g) + ___ O2(g) → ___ CO2(g) + ___ H2O(g)
A)1,1,2,2
B)1,3,2,2
C)2,3,4,2
D)2,2,4,2
A)1,1,2,2
B)1,3,2,2
C)2,3,4,2
D)2,2,4,2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
28
There are approximately 2 × 1022 molecules and atoms in each breath we take and the concentration of CO in the air is approximately 9 parts per million.Approximately how many CO molecules are in each breath we take?
A)2 × 1015
B)1.8 × 1017
C)2 × 1016
D)2 × 1029
A)2 × 1015
B)1.8 × 1017
C)2 × 1016
D)2 × 1029

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
29
Choose the proper coefficients for each substance to yield a balanced equation. 
A)1,1,1
B)2,1,1
C)2,1,2
D)1,1,2

A)1,1,1
B)2,1,1
C)2,1,2
D)1,1,2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
30
The chemical formula for nitrogen monoxide is
A)N2O.
B)NO.
C)NO2.
D)N2O3.
A)N2O.
B)NO.
C)NO2.
D)N2O3.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
31
The name of the compound formed by combining carbon atoms  with oxygen atoms
with oxygen atoms  to form
to form  is
is
A)carbon oxide.
B)monocarbon dioxide.
C)carbon dioxide.
D)carbonate.
 with oxygen atoms
with oxygen atoms  to form
to form  is
isA)carbon oxide.
B)monocarbon dioxide.
C)carbon dioxide.
D)carbonate.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
32
An inversion layer happens when a certain weather pattern traps cooler air near the surface of the earth with a warmer air mass above it.Why is this a problem?
A)Excess precipitation could cause flooding
B)The cold air increases the chance for snowstorms
C)Heatwaves can occur
D)Air pollution concentrates in the inversion layer
A)Excess precipitation could cause flooding
B)The cold air increases the chance for snowstorms
C)Heatwaves can occur
D)Air pollution concentrates in the inversion layer

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
33
Which is the balanced chemical equation showing hydrogen peroxide (H2O2) decomposing into hydrogen (H2) and oxygen (O2)?
A)H2O2 → H2 + O2
B)H2 + O2 → H2O2
C)2 H2 + O2 → 2 H2O2
D)2 H2O2 → 2 H2 + O2
A)H2O2 → H2 + O2
B)H2 + O2 → H2O2
C)2 H2 + O2 → 2 H2O2
D)2 H2O2 → 2 H2 + O2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
34
If we assume that the top of Mt.Everest is the highest land mass on earth,hikers who scale its summit are standing in the
A)mesosphere.
B)stratosphere.
C)troposphere.
D)ozone layer.
A)mesosphere.
B)stratosphere.
C)troposphere.
D)ozone layer.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
35
Catalytic converters reduce the amount of _____ in car exhaust.
A)O3
B)CO2
C)CO
D)N2
A)O3
B)CO2
C)CO
D)N2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
36
Green chemistry is
A)the study of how to improve the production of oxygen via photosynthesis.
B)any chemistry having an agricultural base.
C)the cause of the higher temperatures and humidity typically found in greenhouses.
D)the design of products and processes that reduce hazardous substances.
A)the study of how to improve the production of oxygen via photosynthesis.
B)any chemistry having an agricultural base.
C)the cause of the higher temperatures and humidity typically found in greenhouses.
D)the design of products and processes that reduce hazardous substances.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
37
The lowest (or closest to the ground) layer of our atmosphere is the
A)troposphere.
B)ozone layer.
C)stratosphere.
D)mesosphere.
A)troposphere.
B)ozone layer.
C)stratosphere.
D)mesosphere.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
38
Balance this equation P4 + Cl2 → PCl5 with the smallest whole number coefficients.Choose the answer that is the sum of the coefficients.(Do not forget coefficients of "one.")
A)7
B)9
C)11
D)13
E)15
A)7
B)9
C)11
D)13
E)15

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
39
P2O5 is the chemical formula for
A)pentoxygen diphosphide.
B)diphosphorus pentoxide.
C)dioxygen pentaphosphide.
D)monophosphorus pentoxide.
A)pentoxygen diphosphide.
B)diphosphorus pentoxide.
C)dioxygen pentaphosphide.
D)monophosphorus pentoxide.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
40
Which correctly pairs an indoor pollutant with its source?
A)Formaldehyde and unvented space heaters
B)O3 and electrical arcing
C)Radon and glues and solvents
D)Nicotine and paint and paint thinners
A)Formaldehyde and unvented space heaters
B)O3 and electrical arcing
C)Radon and glues and solvents
D)Nicotine and paint and paint thinners

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
41
What is the chemical formula for carbon disulfide?
A)CH4
B)CS2
C)C2H6
D)H2SO4
A)CH4
B)CS2
C)C2H6
D)H2SO4

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
42
Which chemical components are given off in car exhaust?
A)CO2
B)H2O
C)NOx
D)All of these choices are correct
A)CO2
B)H2O
C)NOx
D)All of these choices are correct

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
43
Currently,the primary source of sulfur dioxide emissions into the atmosphere is
A)coal burning power plants.
B)diesel trucks.
C)plastic manufacturing.
D)gasoline-powered lawnmowers.
A)coal burning power plants.
B)diesel trucks.
C)plastic manufacturing.
D)gasoline-powered lawnmowers.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
44
Even though the concentration of air pollution has gone down over time,people in some metropolitan areas still breath are that contains unhealthy levels of pollutants.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
45
In metropolitan areas,the concentration of ozone in the atmosphere drops at night.Why?
A)Wind blows away the ozone at night
B)Energy usage goes down at night
C)There are less cars on the road at night
D)The formation of ozone requires sunlight
A)Wind blows away the ozone at night
B)Energy usage goes down at night
C)There are less cars on the road at night
D)The formation of ozone requires sunlight

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
46
Which is the correct balanced equation for the complete combustion of ethane in excess oxygen?
A)CH4 + 2 O2 → CO2 + 2 H2O
B)2 CH4 + 3 O2 → 2 CO + 4 H2O
C)2C2H6 + 7 O2 → 4 CO2 + 6 H2O
D)2 C2H6 + 5 O2 → 4 CO + 6 H2O
A)CH4 + 2 O2 → CO2 + 2 H2O
B)2 CH4 + 3 O2 → 2 CO + 4 H2O
C)2C2H6 + 7 O2 → 4 CO2 + 6 H2O
D)2 C2H6 + 5 O2 → 4 CO + 6 H2O

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
47
Which air pollutant is the second-leading cause of lung cancer worldwide,behind tobacco smoke?
A)Radon
B)Ozone
C)Carbon monoxide
D)Nitrogen oxides
A)Radon
B)Ozone
C)Carbon monoxide
D)Nitrogen oxides

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
48
What is the greatest source of indoor air pollution in developing countries?
A)Unvented space heaters
B)Cookstoves
C)Automobiles
D)Paint
A)Unvented space heaters
B)Cookstoves
C)Automobiles
D)Paint

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
49
The more toxic the pollutant,the higher the concentration is set when concerning air quality standards.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
50
How many carbon atoms does the molecule octane have?
A)0
B)2
C)4
D)8
A)0
B)2
C)4
D)8

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck



