Deck 1: Portable Electronics: the Periodic Table in the Palm of Your Hand
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/50
Play
Full screen (f)
Deck 1: Portable Electronics: the Periodic Table in the Palm of Your Hand
1
Which symbol(s) represent(s) elements in the noble gas family? 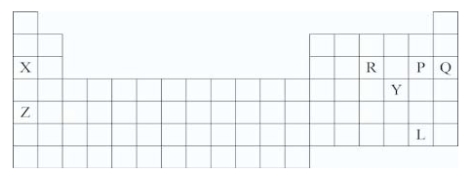
A)X and Z
B)P and L
C)Q
D)Y

A)X and Z
B)P and L
C)Q
D)Y
Q
2
A(n) ________ is a fixed number of atoms held together by chemical bonds in a certain spatial arrangement.
A)element
B)ion
C)molecule
D)mixture
A)element
B)ion
C)molecule
D)mixture
molecule
3
Which diagram(s) best represent(s) only individual atoms? 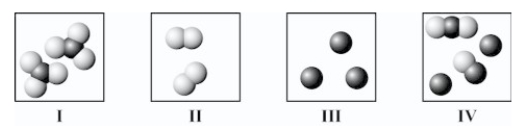
A)I only
B)II only
C)III only
D)IV only
E)II and III only

A)I only
B)II only
C)III only
D)IV only
E)II and III only
III only
4
On a Periodic Table,the columns of elements with similar properties are
A)periods.
B)groups.
C)rows.
D)metals.
A)periods.
B)groups.
C)rows.
D)metals.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is a pure substance?
A)Lemonade
B)Concrete
C)Gasoline
D)Silver wire
A)Lemonade
B)Concrete
C)Gasoline
D)Silver wire

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
6
The quantity 0.0000064 g expressed in scientific notation.
A)6.4 × 106 g
B)6.4 × 10¯6 g
C)6.4 × 107 g
D)6.4 × 10¯7 g
A)6.4 × 106 g
B)6.4 × 10¯6 g
C)6.4 × 107 g
D)6.4 × 10¯7 g

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
7
Which is not a pure substance?
A)Helium
B)Copper wire
C)Air
D)Sucrose
A)Helium
B)Copper wire
C)Air
D)Sucrose

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
8
Which diagram(s) best represent(s) only molecules? 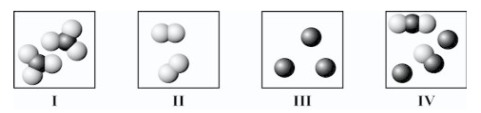
A)I only
B)II only
C)III only
D)I and II only
E)IV only

A)I only
B)II only
C)III only
D)I and II only
E)IV only

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
9
Which square(s) contain(s) only one or more compounds? 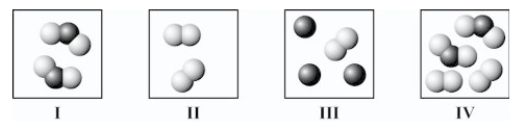
A)I only
B)II only
C)I and IV only
D)II and III only

A)I only
B)II only
C)I and IV only
D)II and III only

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
10
Which square(s) contain(s) only an element? 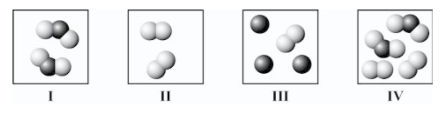
A)I only
B)II only
C)I and II only
D)III and IV only

A)I only
B)II only
C)I and II only
D)III and IV only

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
11
Which substance is an element?
A)NO2
B)NaCl
C)N2
D)CH4
A)NO2
B)NaCl
C)N2
D)CH4

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
12
The quantity 8.7 × 105 g expressed in standard decimal notation.
A)0.000087 g
B)870.000 g
C)0.0000087 g
D)870,000 g
A)0.000087 g
B)870.000 g
C)0.0000087 g
D)870,000 g

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
13
Which symbols represent only elements that are metals? 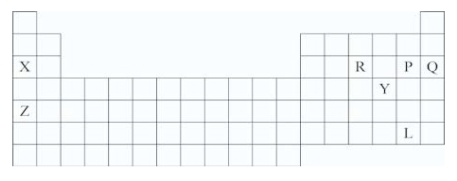
A)X and Z
B)X and Q
C)P and L
D)X,R,P,and Q

A)X and Z
B)X and Q
C)P and L
D)X,R,P,and Q

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
14
A substance that can be broken down into two or more simpler substances by chemical methods is called a(n)
A)compound.
B)mixture.
C)element.
D)isotope.
A)compound.
B)mixture.
C)element.
D)isotope.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
15
Which diagram(s) best represent(s) only diatomic molecules? 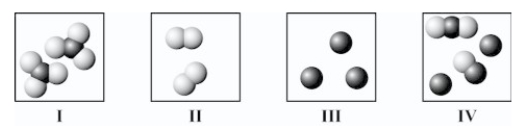
A)I only
B)II only
C)I and II only
D)II and IV only

A)I only
B)II only
C)I and II only
D)II and IV only

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
16
Which squares contain mixtures? 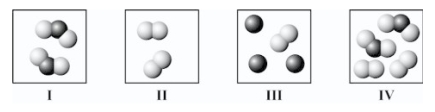
A)II and III only
B)III and IV only
C)I,III,and IV only
D)I and IV only

A)II and III only
B)III and IV only
C)I,III,and IV only
D)I and IV only

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
17
Which differentiates a compound from a mixture of two or more elements?
A)The elements in a compound may be present in varying proportions
B)A compound does not exhibit the individual properties of the elements of which it is composed
C)A compound is made up of only one element
D)A compound cannot be made up of more than two elements
A)The elements in a compound may be present in varying proportions
B)A compound does not exhibit the individual properties of the elements of which it is composed
C)A compound is made up of only one element
D)A compound cannot be made up of more than two elements

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is the chemical symbol for silver?
A)Au
B)Pb
C)Ag
D)Fe
A)Au
B)Pb
C)Ag
D)Fe

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
19
Which is not a mixture?
A)A jar filled with rocks and sand
B)Sea water
C)A glass of Kool-Aid
D)Sodium chloride
A)A jar filled with rocks and sand
B)Sea water
C)A glass of Kool-Aid
D)Sodium chloride

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
20
The most numerous of the elements are the
A)metals.
B)nonmetals.
C)metalloids.
D)noble gases.
A)metals.
B)nonmetals.
C)metalloids.
D)noble gases.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
21
Which corresponds to the composition of the ion typically formed by magnesium? 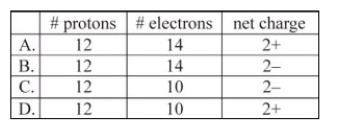
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
22
Which one of these materials is most likely to conduct heat?
A)A titanium rod
B)A sample of chlorine gas
C)A silicon wafer
D)A chunk of phosphorus
A)A titanium rod
B)A sample of chlorine gas
C)A silicon wafer
D)A chunk of phosphorus

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
23
What type of material is defined as having a well-ordered atomic structure?
A)Amorphous
B)Crystalline
C)Transparent
D)Opaque
A)Amorphous
B)Crystalline
C)Transparent
D)Opaque

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
24
Which one of the following is an example of a homogeneous mixture?
A)A chocolate chip cookie
B)A glass of pulp-free lemonade
C)A carbonated beverage
D)A bowl of cereal in milk
A)A chocolate chip cookie
B)A glass of pulp-free lemonade
C)A carbonated beverage
D)A bowl of cereal in milk

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
25
Which country is the world's leading producer of rare earth elements?
A)USA
B)China
C)South Africa
D)Brazil
A)USA
B)China
C)South Africa
D)Brazil

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
26
The numbers 1 through 4 are used to identify four different elements on the periodic table.Which element is expected to have 2+ charge when it forms an ion? 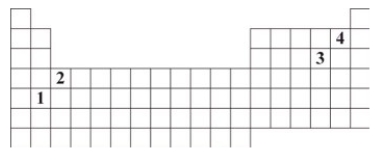
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
27
The majority of new smartphones being released have displays that are at least 5 inches in size.What is this size in centimeters? (There are 2.54 cm in one inch. )
A)1.97 cm
B)6.10 cm
C)12.7 cm
D)60.0 cm
A)1.97 cm
B)6.10 cm
C)12.7 cm
D)60.0 cm

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
28
In the periodic table,which elements typically have similar properties?
A)Those in the same rows
B)Those related diagonally
C)Those in the same columns
D)Those on opposite sides
A)Those in the same rows
B)Those related diagonally
C)Those in the same columns
D)Those on opposite sides

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
29
What distinguishes the atoms of one element from another?
A)The number of neutrons
B)The number of protons plus neutrons
C)The number of protons
D)The number of neutrons plus electrons
A)The number of neutrons
B)The number of protons plus neutrons
C)The number of protons
D)The number of neutrons plus electrons

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
30
A recent set of advertisements has a picture of an aluminum can with an aluminum-frame bicycle,along with a hypothetical quote from the can that reads: "I want to be a bike.Recycle me." The analysis of the life cycle of an item starting with its raw materials and ending with the used item becoming the raw material for new products is called
A)cradle-to-grave.
B)reusability.
C)cradle-to-cradle.
D)cost-benefit analysis.
A)cradle-to-grave.
B)reusability.
C)cradle-to-cradle.
D)cost-benefit analysis.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
31
How many protons,neutrons,and electrons are there in a neutral atom of 19/9 F? 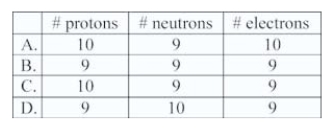
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
32
What are the three pillars of sustainability?
A)Reduce,reuse,recycle
B)Equitability,justice,quality of life
C)Diversity,efficiency,toxicity
D)Environmental,social,economic
A)Reduce,reuse,recycle
B)Equitability,justice,quality of life
C)Diversity,efficiency,toxicity
D)Environmental,social,economic

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
33
One common class of minerals are aluminosilicates,with a general formula of Al2SiO5.What is the atomic percentage composition of aluminum in an aluminosilicate?
A)12.5 percent Al
B)25 percent Al
C)33.3 percent Al
D)62.5 percent Al
A)12.5 percent Al
B)25 percent Al
C)33.3 percent Al
D)62.5 percent Al

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
34
Which one of the following compounds is considered to be an ionic compound?
A)NO
B)CCl4
C)LiF
D)PI3
A)NO
B)CCl4
C)LiF
D)PI3

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
35
Which corresponds to the composition of the ion typically formed by fluorine? 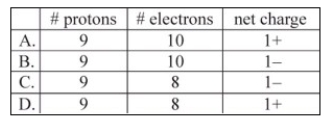
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
36
How many bromide ions can combine with one ion of aluminum to form an ionic compound?
A)3
B)6
C)1
D)2
A)3
B)6
C)1
D)2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
37
Which one of the following compounds is considered to be a molecular compound?
A)PCl3
B)NaBr
C)Al2O3
D)Mg3P2
A)PCl3
B)NaBr
C)Al2O3
D)Mg3P2

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
38
Which one of the following is a subatomic particle located in the center of the atom and has a positive charge?
A)Protons
B)Neutrons
C)Electrons
D)Photons
A)Protons
B)Neutrons
C)Electrons
D)Photons

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
39
The atomic number is the
A)same as the mass number of an atom.
B)number of protons in a nucleus.
C)number of protons and neutrons in a nucleus.
D)number of neutrons in a nucleus.
A)same as the mass number of an atom.
B)number of protons in a nucleus.
C)number of protons and neutrons in a nucleus.
D)number of neutrons in a nucleus.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
40
The nucleus of an atom contains
A)electrons and protons only.
B)protons only.
C)electrons,protons,and neutrons.
D)protons and neutrons only.
A)electrons and protons only.
B)protons only.
C)electrons,protons,and neutrons.
D)protons and neutrons only.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
41
A sample of silicon rejected for use in an electronic circuit has an impurity of boron at a level of 3 parts per trillion.What is the fraction of boron atoms in the silicon sample as written in scientific notation?
A)3 x 10-9
B)3 x 109
C)3 x 10-12
D)3 x 106
A)3 x 10-9
B)3 x 109
C)3 x 10-12
D)3 x 106

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
42
One of the lightest smartphones on the market today weighs 113 g.To protect your phone,you may want to use a screen protector,which weighs 27.2 g,and a heavy duty phone case,which weighs 114 g.What is the total weight of the phone,screen protector,and case?
A)254.20 g
B)254.2 g
C)254 g
D)250 g
A)254.20 g
B)254.2 g
C)254 g
D)250 g

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
43
What is the term used to describe a material that allows light to mostly pass through it?
A)Reflective
B)Porous
C)Transparent
D)Absorbtive
A)Reflective
B)Porous
C)Transparent
D)Absorbtive

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
44
What is the answer to the following mathematical operation? 1.42 + 9.4 x 2.854
A)30.8
B)30.82
C)30.88028
D)31
A)30.8
B)30.82
C)30.88028
D)31

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
45
How many cm are in 0.129 m?
A)1.29 cm
B)12.9 cm
C)129 cm
D)1290 cm
A)1.29 cm
B)12.9 cm
C)129 cm
D)1290 cm

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
46
How many protons are in an atom of carbon?
A)2
B)4
C)6
D)12
A)2
B)4
C)6
D)12

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
47
What term is defined as the flow of electrons from one location to another?
A)Resistance
B)Transparency
C)Density
D)Electricity
A)Resistance
B)Transparency
C)Density
D)Electricity

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
48
What is the density of a block of material that is 12 cm x 6 cm x 2 cm and has a mass of 356 g?
A)1.00 g/cm3
B)2.47 g/cm3
C)3.56 g/cm3
D)4.95 g/cm3
A)1.00 g/cm3
B)2.47 g/cm3
C)3.56 g/cm3
D)4.95 g/cm3

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
49
A computer chip was weighed and found to have a mass of 23.3040 g.How many significant figures does this number have?
A)4
B)5
C)6
D)7
A)4
B)5
C)6
D)7

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
50
Oxidation and reduction of atoms involves a change in the number of ___________ in the atom.
A)electrons
B)protons
C)neutrons
D)nuclei
A)electrons
B)protons
C)neutrons
D)nuclei

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck



