Deck 23: Mass Spectrometry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 23: Mass Spectrometry
1
The resolving power for a mass spectrometer can be calculated using the equations 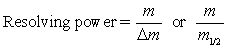 Which of the following statements are NOT true regarding resolving power?
Which of the following statements are NOT true regarding resolving power?
I m is the larger value of m/z
II m is the difference between the two m/z peaks
III m1/2 is the width of the peak at half maximum height
IV m is the smaller value of m/z
V m1/2 is the average mass for the two peaks
A)I and V
B)II,III,and IV
C)I,II,and III
D)I,II,and V
E)II and IV
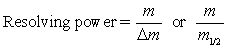 Which of the following statements are NOT true regarding resolving power?
Which of the following statements are NOT true regarding resolving power?I m is the larger value of m/z
II m is the difference between the two m/z peaks
III m1/2 is the width of the peak at half maximum height
IV m is the smaller value of m/z
V m1/2 is the average mass for the two peaks
A)I and V
B)II,III,and IV
C)I,II,and III
D)I,II,and V
E)II and IV
I and V
2
Calculate the resolving power for m/z = 283.980 if the width of half height is 0.076 m/z.
3737
3
Which is true for mass spectrometry of proteins?
I MALDI and electrospray are major methods for introducing proteins into the gas phase.
II The equation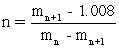 is used to determine the number of H+ bound to a protein from the mass spectrum.
is used to determine the number of H+ bound to a protein from the mass spectrum.
III Once n is known;the molecular mass of the protein is calculated,molecular mass = nmn.
IV Electron-transfer dissociation is a mass spectrometry technique to sequence proteins.
A)I,II,and IV
B)II and IV
C)III and IV
D)I,III,and IV
E)II and III
I MALDI and electrospray are major methods for introducing proteins into the gas phase.
II The equation
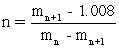 is used to determine the number of H+ bound to a protein from the mass spectrum.
is used to determine the number of H+ bound to a protein from the mass spectrum.III Once n is known;the molecular mass of the protein is calculated,molecular mass = nmn.
IV Electron-transfer dissociation is a mass spectrometry technique to sequence proteins.
A)I,II,and IV
B)II and IV
C)III and IV
D)I,III,and IV
E)II and III
I,II,and IV
4
Calculate the resolving power for a mass spectrometer that successfully separates m/z 125.135 from 125.293.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
Which is NOT a chromatography-mass spectrometry interface?
A)electrospray
B)direct electron ionization
C)matrix-assisted laser desorption ionization
D)atmospheric pressure chemical ionization
E)photoionization
A)electrospray
B)direct electron ionization
C)matrix-assisted laser desorption ionization
D)atmospheric pressure chemical ionization
E)photoionization

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
Which is true for using mass spectroscopy as a chromatography detector?
I Selected ion monitoring observes only one to four m/z to create a selected ion chromatogram.
II Extracted ion chromatogram records the entire mass spectrum for the compounds coming off the column,picking one m/z from the collected mass spectra to create the chromatogram.
III Reconstructed total ion chromatogram records the total ion current below a certain m/z to generate the chromatogram.
IV Selected reaction monitoring creates a chromatogram using a triple quadrupole mass spectrometer.Quadrupole 1 selects the precursor ion.In quadrupole 2,the precursor ion is fragmented through collisions with N2 or Ar gas.All fragment ions pass to quadrupole 3,which selects one ion fragment to monitor.
A)I,II,III,and IV
B)II,III,and IV
C)I and IV
D)IV only
E)I,II,and IV
I Selected ion monitoring observes only one to four m/z to create a selected ion chromatogram.
II Extracted ion chromatogram records the entire mass spectrum for the compounds coming off the column,picking one m/z from the collected mass spectra to create the chromatogram.
III Reconstructed total ion chromatogram records the total ion current below a certain m/z to generate the chromatogram.
IV Selected reaction monitoring creates a chromatogram using a triple quadrupole mass spectrometer.Quadrupole 1 selects the precursor ion.In quadrupole 2,the precursor ion is fragmented through collisions with N2 or Ar gas.All fragment ions pass to quadrupole 3,which selects one ion fragment to monitor.
A)I,II,III,and IV
B)II,III,and IV
C)I and IV
D)IV only
E)I,II,and IV

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
Calculate the number of rings and bonds for the chemical formula C23H28N2O5S2.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
When identifying the molecular ion peak, 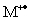 ,all of the following must be kept in mind,except:
,all of the following must be kept in mind,except:
A)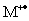
Will be the highest m/z value of any of the "significant" peaks in the spectrum that can not be attributed to isotopes or background.
B)intensities of isotopic peaks M+1,M+2 and so forth must be consistent with the proposed formula.
C)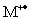
Will be at least 70% of the base peak.
D)the peak for the heaviest fragment ion should not correspond to an improbable mass loss from
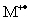
)
E)if a fragment ion is known to contain X atoms of element Z,then there must be at least X atoms of element Z in the molecular ion.
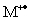 ,all of the following must be kept in mind,except:
,all of the following must be kept in mind,except:A)
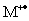
Will be the highest m/z value of any of the "significant" peaks in the spectrum that can not be attributed to isotopes or background.
B)intensities of isotopic peaks M+1,M+2 and so forth must be consistent with the proposed formula.
C)
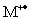
Will be at least 70% of the base peak.
D)the peak for the heaviest fragment ion should not correspond to an improbable mass loss from
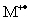
)
E)if a fragment ion is known to contain X atoms of element Z,then there must be at least X atoms of element Z in the molecular ion.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
The three essential components of any mass spectrometer are:
I ion source.
II decelerator.
III detector.
IV mass separator.
V accelerator.
A)I,III,and IV
B)II,III,and IV
C)I,III,and III
D)II,IV,and V
E)III,IV,and IV
I ion source.
II decelerator.
III detector.
IV mass separator.
V accelerator.
A)I,III,and IV
B)II,III,and IV
C)I,III,and III
D)II,IV,and V
E)III,IV,and IV

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
Which statements are true for the various ion selection techniques?
I Time-of-flight MS - Ions expelled from the source all have the same kinetic energy.For ions of different masses but equivalent kinetic energies,the lighter ions travel faster than heavier ions,separating the ions.
II Quadrupole MS - A constant voltage and a radio-frequency oscillating voltage is applied to the four metal rods.The electric field deflects ions in complex trajectories as the ions migrate from source to detector,allowing only one particular m/z to reach the detector.
III Three-dimensional quadrupole ion-trap MS - A constant-frequency radio-frequency voltage is applied to the central ring electrode causes ions to circulate in stable,three-dimensional orbits in the cavity,with the lowest m/z in the outermost orbits.Increasing the amplitude of the radio-frequency voltage destabilizes m/z ion orbits one value at a time.
IV Magnetic sector MS - Ions are accelerated into the magnetic sector with equivalent kinetic energies.Ions are selected by passing through a magnetic field;lighter ions are deflected the most and the heavier ions are deflected the least.The magnetic field is varied to select individual m/z.
A)I,II,and IV
B)I,II,III,and IV
C)II,III,and IV
D)II and IV
E)III and IV
I Time-of-flight MS - Ions expelled from the source all have the same kinetic energy.For ions of different masses but equivalent kinetic energies,the lighter ions travel faster than heavier ions,separating the ions.
II Quadrupole MS - A constant voltage and a radio-frequency oscillating voltage is applied to the four metal rods.The electric field deflects ions in complex trajectories as the ions migrate from source to detector,allowing only one particular m/z to reach the detector.
III Three-dimensional quadrupole ion-trap MS - A constant-frequency radio-frequency voltage is applied to the central ring electrode causes ions to circulate in stable,three-dimensional orbits in the cavity,with the lowest m/z in the outermost orbits.Increasing the amplitude of the radio-frequency voltage destabilizes m/z ion orbits one value at a time.
IV Magnetic sector MS - Ions are accelerated into the magnetic sector with equivalent kinetic energies.Ions are selected by passing through a magnetic field;lighter ions are deflected the most and the heavier ions are deflected the least.The magnetic field is varied to select individual m/z.
A)I,II,and IV
B)I,II,III,and IV
C)II,III,and IV
D)II and IV
E)III and IV

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
Analytes must be ionized prior to entering the mass filter in a mass spectrometer.Electron ionization and chemical ionization are two ionization techniques.Which of the following statements are INCORRECT regarding either technique?
I Electron ionization uses an electron beam to create molecular ions,M+ . .
II Chemical ionization uses an electron beam to ionize reagent gas.
III Electron ionization gives very little fragmentation of the molecular ion.
IV The ionized reagent gas in chemical ionization undergoes a complex set of chemical reactions before protonating the analyte to create MH+.
V Both ionization techniques give identical mass spectra.
A)I,III,and V
B)III and V
C)II,III,and IV
D)I,II,and IV
E)I,II,and V
I Electron ionization uses an electron beam to create molecular ions,M+ . .
II Chemical ionization uses an electron beam to ionize reagent gas.
III Electron ionization gives very little fragmentation of the molecular ion.
IV The ionized reagent gas in chemical ionization undergoes a complex set of chemical reactions before protonating the analyte to create MH+.
V Both ionization techniques give identical mass spectra.
A)I,III,and V
B)III and V
C)II,III,and IV
D)I,II,and IV
E)I,II,and V

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
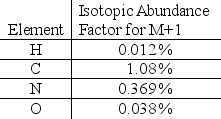
The mass spectrum of an unknown compound has a molecular ion peak at 194 m/z.The proposed chemical formula for the compound is C8H10N4O2.The M+1 peak has a relative abundance of 10.1%.Does the intensity of the M+1 peak support the proposed chemical formula?


Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
Electrospray ionization is one of many techniques interfacing chromatography with mass spectroscopy.Which is NOT true for electrospray ionization?
A)The steel nebulizer is held at 0 V while the spray chamber is held at −3500 V.
B)The strong electric field between the nebulizer and spray chamber creates a fine aerosol.
C)The glass capillary leading to the mass spectrometer is held at a potential of −4500 V to attract the gas phase cations.
D)Typically the ions that vaporize from aerosol droplets were already charged while on the chromatography column.
E)There is minimal fragmentation,but fragmentation can be increased by collisionally activated dissociation.
A)The steel nebulizer is held at 0 V while the spray chamber is held at −3500 V.
B)The strong electric field between the nebulizer and spray chamber creates a fine aerosol.
C)The glass capillary leading to the mass spectrometer is held at a potential of −4500 V to attract the gas phase cations.
D)Typically the ions that vaporize from aerosol droplets were already charged while on the chromatography column.
E)There is minimal fragmentation,but fragmentation can be increased by collisionally activated dissociation.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
Which is NOT true for ion mobility spectrometry?
A)Ion mobility spectrometer is not a form of mass spectrometry.
B)Ion mobility spectrometry is gas-phase electrophoresis.
C)Ions are separated according to their size-to-charge ratio.
D)Ions are generated when sample is desorbed and passes through a 10 millilcurie 63Ni source.
E)All ions travel with the same kinetic energy.
A)Ion mobility spectrometer is not a form of mass spectrometry.
B)Ion mobility spectrometry is gas-phase electrophoresis.
C)Ions are separated according to their size-to-charge ratio.
D)Ions are generated when sample is desorbed and passes through a 10 millilcurie 63Ni source.
E)All ions travel with the same kinetic energy.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
Calculate the number of rings and double bonds for the chemical formula C25H32N2O3.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
Ion mobility spectrometry________________________________________and____________________ .
A)operates at ambient pressure;is gas-phase electrophoresis
B)operates at low pressure;is gas-phase electrophoresis
C)operates at ambient pressure;is a form of mass spectrometry
D)is gas-phase electrophoresis;is a form of mass spectrometry
E)operates at ambient pressure;high pressure
A)operates at ambient pressure;is gas-phase electrophoresis
B)operates at low pressure;is gas-phase electrophoresis
C)operates at ambient pressure;is a form of mass spectrometry
D)is gas-phase electrophoresis;is a form of mass spectrometry
E)operates at ambient pressure;high pressure

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
Which is NOT true of mass spectrometers?
A)Double-focusing mass spectrometers couple an electric sector to a magnetic sector to increase the resolution to ~105.
B)Transmission quadrupole mass spectrometers select ions by applying a constant voltage and a radio-frequency oscillating voltage to the four parallel metal rods.
C)Orbitrap mass spectrometers do not require magnetic or radio-frequency fields to measure m/z.
D)Quadrupole mass spectrometers operate at constant resolving power.
E)The time-of-flight mass spectrometer employs a reflectron to group ions with the same m/z,regardless of initial kinetic energy.
A)Double-focusing mass spectrometers couple an electric sector to a magnetic sector to increase the resolution to ~105.
B)Transmission quadrupole mass spectrometers select ions by applying a constant voltage and a radio-frequency oscillating voltage to the four parallel metal rods.
C)Orbitrap mass spectrometers do not require magnetic or radio-frequency fields to measure m/z.
D)Quadrupole mass spectrometers operate at constant resolving power.
E)The time-of-flight mass spectrometer employs a reflectron to group ions with the same m/z,regardless of initial kinetic energy.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
The molecular ion peak for a compound is 180 Da.The number of nitrogen atoms in the compound can be:
A)an odd number of nitrogens.
B)an even number of nitrogens.
C)zero nitrogens.
D)A and C
E)B and C
A)an odd number of nitrogens.
B)an even number of nitrogens.
C)zero nitrogens.
D)A and C
E)B and C

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
How many rings and double bonds are there for the compound C11H7NO2Cl2?
A)c = 10;h = 7;n = 1;R&DB = 8
B)c = 11;h = 9;n = 1;R&DB = 8
C)c = 10;h = 7;n = 3;R&DB = 9
D)c = 11;h = 7;n = 1;R&DB = 9
E)c = 11;h = 9;n = 3;R&DB = 9
A)c = 10;h = 7;n = 1;R&DB = 8
B)c = 11;h = 9;n = 1;R&DB = 8
C)c = 10;h = 7;n = 3;R&DB = 9
D)c = 11;h = 7;n = 1;R&DB = 9
E)c = 11;h = 9;n = 3;R&DB = 9

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
Analytes on the surface of an object can be sampled using an open-air sampling technique by vaporizing and ionizing the analyte directly from the surface under atmospheric conditions.Which of the following statements are true for these techniques?
I Direct analysis in real time generates excited He which produces protonated water clusters from distilled water.The water clusters protonate the analyte.
II Low-temperature plasma generates excited-state species in plasma,which ionizes the analyte.
III Desorption electrospray ionization directs micron-sized droplets of analyte-free solvent with electrospray,dissolving the analyte in the droplets.
IV All techniques dislodge analyte from the surface,collecting the ions for later mass spectrometer analysis.
A)I and IV
B)II and III
C)I and III
D)II and IV
E)I and II
I Direct analysis in real time generates excited He which produces protonated water clusters from distilled water.The water clusters protonate the analyte.
II Low-temperature plasma generates excited-state species in plasma,which ionizes the analyte.
III Desorption electrospray ionization directs micron-sized droplets of analyte-free solvent with electrospray,dissolving the analyte in the droplets.
IV All techniques dislodge analyte from the surface,collecting the ions for later mass spectrometer analysis.
A)I and IV
B)II and III
C)I and III
D)II and IV
E)I and II

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck



