Deck 27: Chromatographic Methods and Capillary Electrophoresis
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 27: Chromatographic Methods and Capillary Electrophoresis
1
Which statement regarding capillary electrophoresis sample injection is INCORRECT?
A)Hydrodynamic injection uses a pressure differential between the two ends of the capillary to inject a volume of sample onto the capillary.
B)Electrokinetic injection uses an electric field to inject a volume of sample onto the capillary.
C)For electrokinetic injection,each analyte has a different app,so the composition of the loaded sample is different from the original sample.
D)For quantitative work,an internal standard is essential because the amount of sample injected is not reproducible between runs.
E)Stacking concentrates the sample cations and anions in the sample at the interfaces between the sample plug and run buffer.
A)Hydrodynamic injection uses a pressure differential between the two ends of the capillary to inject a volume of sample onto the capillary.
B)Electrokinetic injection uses an electric field to inject a volume of sample onto the capillary.
C)For electrokinetic injection,each analyte has a different app,so the composition of the loaded sample is different from the original sample.
D)For quantitative work,an internal standard is essential because the amount of sample injected is not reproducible between runs.
E)Stacking concentrates the sample cations and anions in the sample at the interfaces between the sample plug and run buffer.
Electrokinetic injection uses an electric field to inject a volume of sample onto the capillary.
2
The retention time for an analyte separated via capillary electrophoresis is 115 s.The separation occurred with a 50-cm column,with the detector at 45 cm,and a potential of 30 kV.A neutral molecule took 200 s to reach the detector.What is the electrophoretic mobility for the analyte?
ep = 2.77 × 10−8 m2/Vs
3
What impact does the pH of the mobile phase have on the ability of a strongly acidic exchanger to interact with ions?
A)decreases the ability to interact with ions as pH decreases
B)increases the ability to interact with ions as pH increases
C)has no impact on the ability to interact with ions
D)decreases the ability to interact with ions as pH increases
E)increases the ability to interact with ions as pH decreases
A)decreases the ability to interact with ions as pH decreases
B)increases the ability to interact with ions as pH increases
C)has no impact on the ability to interact with ions
D)decreases the ability to interact with ions as pH increases
E)increases the ability to interact with ions as pH decreases
has no impact on the ability to interact with ions
4
________________________________________occurs when an electric field is applied to a capillary that has an electric double layer at the negatively charged surface inside the capillary.
A)Electropsmotic flow
B)Electrophoretic flow
C)Electrodynamic flow
D)Electrodiffusion flow
E)Electroeffusion flow
A)Electropsmotic flow
B)Electrophoretic flow
C)Electrodynamic flow
D)Electrodiffusion flow
E)Electroeffusion flow

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
Which is NOT a detector used with capillary electrophoresis?
A)ultraviolet absorption
B)conductivity
C)electrospray mass spectrometry
D)laser induced fluorescence
E)evaporative light scattering
A)ultraviolet absorption
B)conductivity
C)electrospray mass spectrometry
D)laser induced fluorescence
E)evaporative light scattering

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
The normal order for elution in capillary zone electrophoresis is:
A)anions (highest mobility last),all neutrals (unseparated),cations (highest mobility first).
B)anions (lowest mobility first),all neutrals (unseparated),cations (lowest mobility first).
C)cations (highest mobility first),all neutrals (unseparated),anions (highest mobility last).
D)cations (lowest mobility first),all neutrals (unseparateD),anions (lowest mobility last).
E)cations (highest mobility first),neutrals (lowest mobility first),anions (highest mobility first).
A)anions (highest mobility last),all neutrals (unseparated),cations (highest mobility first).
B)anions (lowest mobility first),all neutrals (unseparated),cations (lowest mobility first).
C)cations (highest mobility first),all neutrals (unseparated),anions (highest mobility last).
D)cations (lowest mobility first),all neutrals (unseparateD),anions (lowest mobility last).
E)cations (highest mobility first),neutrals (lowest mobility first),anions (highest mobility first).

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
Sodium cation was separated from a mixture of cations via capillary electrophoresis,with negative detection.The instrument is equipped with a capillary 50 cm in length,with 45 cm to the detector.The separation took place under 30 kV with the neutral marker reaching the detector after 200 seconds.The retention time for Na+ is 85 seconds.Calculate the number of theoretical plates for the Na+ peak.The diffusion coefficient for Na+ in water is 1.0 × 10−9 m2/s.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
Which statement is NOT true for hydrophobic interaction chromatography?
A)Hydrophobic interaction chromatography has a hydrophobic stationary phase,which repels water and is not wetted by water.The technique is used primarily for protein purification.
B)Proteins are salted out of solution with ammonium,sodium,and potassium salts of phosphate and sulfate.Thiocyanate,iodide,and perchlorate salts increase the solubility of proteins in water.
C)The proteins are eluted from the column with a gradient of increasing salt concentration,increasing the solubility of proteins in water.
D)The most common stationary phase is an agarose gel with hydrophobic phenyl or alkyl groups attached and a pore size large enough to accommodate proteins.
E)A protein sample with a large concentration of ammonium sulfate is applied to the column.The ammonium sulfate causes the protein to stick to the hydrophobic surface of the stationary phase.
A)Hydrophobic interaction chromatography has a hydrophobic stationary phase,which repels water and is not wetted by water.The technique is used primarily for protein purification.
B)Proteins are salted out of solution with ammonium,sodium,and potassium salts of phosphate and sulfate.Thiocyanate,iodide,and perchlorate salts increase the solubility of proteins in water.
C)The proteins are eluted from the column with a gradient of increasing salt concentration,increasing the solubility of proteins in water.
D)The most common stationary phase is an agarose gel with hydrophobic phenyl or alkyl groups attached and a pore size large enough to accommodate proteins.
E)A protein sample with a large concentration of ammonium sulfate is applied to the column.The ammonium sulfate causes the protein to stick to the hydrophobic surface of the stationary phase.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
The commonly used detector with ion chromatography is the:
A)conductivity detector.
B)fluorescence detector.
C)ion detector.
D)gravimetric detector.
E)oxidation detector.
A)conductivity detector.
B)fluorescence detector.
C)ion detector.
D)gravimetric detector.
E)oxidation detector.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
____________________ is used to isolate a single compound or class of compounds from a complex mixture by passing the sample through a column where only one solute is bound.The remaining solutes pass through the column.The bound solute is eluted from the column by changing a condition such as________________________________________ to weaken its binding.
A)Adhesion chromatography;ratio of polar and nonpolar solvents
B)Binding chromatography;pH or ionic strength
C)Affinity chromatography;the polarity of the solvent
D)Adhesion chromatography;the temperature of the column
E)Affinity chromatography;pH or ionic strength
A)Adhesion chromatography;ratio of polar and nonpolar solvents
B)Binding chromatography;pH or ionic strength
C)Affinity chromatography;the polarity of the solvent
D)Adhesion chromatography;the temperature of the column
E)Affinity chromatography;pH or ionic strength

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
Capillary electrophoresis separates two analytes with retention times of 112 s and 113 s with a 60 cm capillary,with the detector 50 cm from the inlet.The voltage applied across the capillary is 20 kV.Assuming that the peaks eluted close enough that N is essentially constant,what is the resolution for the two peaks if N = 500 000 plates?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
A sample of NaNO3 and K2SO4 is separated on a suppressed-ion anion chromatography column.________________________________________is retained and separated on the separator column.________________________________________is used to elute the analyte from the separator column.____________________ is removed in the suppressor to reduce____________________ and allow for detection of analyte.
A)Na+ and K+ HCl NO3− and SO42− Background absorbance
B)NO3− and SO42− HCl Na+ and K+ Background conductivity
C)Na+ and K+ KOH NO3− and SO42− Background conductivity
D)NO3− and SO42− KOH Na+ and K+____________________Background absorbance
E)None of the above is correct.
A)Na+ and K+ HCl NO3− and SO42− Background absorbance
B)NO3− and SO42− HCl Na+ and K+ Background conductivity
C)Na+ and K+ KOH NO3− and SO42− Background conductivity
D)NO3− and SO42− KOH Na+ and K+____________________Background absorbance
E)None of the above is correct.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
____________________ are instruments constructed on plastic or glass chips which use electroosmotic flow or pressure to move liquids through micrometer channels from one reaction site to another.
A)Microosmotic devices
B)Microcapillary devices
C)Microelectrophoric devices
D)Microfluidic devices
E)Microscale devices
A)Microosmotic devices
B)Microcapillary devices
C)Microelectrophoric devices
D)Microfluidic devices
E)Microscale devices

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
Which statements are true for molecular exclusion chromatography?
I Molecular exclusion chromatography separates on the basis of size.
II Small molecules that freely pass through the stationary phase,Kav = 0.
III Small molecules can fit in the pores of the stationary phase and effectively pass through a larger volume,and elute last.
IV The pores in the stationary phase are too small for large molecules to pass through,so large molecules elute first.
V Molecular exclusion chromatography with a hydrophilic stationary phase and an aqueous solvent is called gel permeation chromatography.
A)I,II,III,and V
B)II and V
C)I,II,and IV
D)I,III,and IV
E)III,IV,and V
I Molecular exclusion chromatography separates on the basis of size.
II Small molecules that freely pass through the stationary phase,Kav = 0.
III Small molecules can fit in the pores of the stationary phase and effectively pass through a larger volume,and elute last.
IV The pores in the stationary phase are too small for large molecules to pass through,so large molecules elute first.
V Molecular exclusion chromatography with a hydrophilic stationary phase and an aqueous solvent is called gel permeation chromatography.
A)I,II,III,and V
B)II and V
C)I,II,and IV
D)I,III,and IV
E)III,IV,and V

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
Capillary columns require conditioning prior to their first use.Typical conditioning is washing with 1 M NaOH for 1 hour,followed by 1 hour of water,then 1 hour of 6 M HCl,and finally 1 hour of run buffer.Which statements are INCORRECT?
I The NaOH wash is thought to generate the Si-OH groups on the silica surface.
II The NaOH wash removes any acidic contaminants from the silica surface.
III The HCl wash is thought to generate the Si-OH groups on the silica surface.
IV The HCl wash removes metal ions from the capillary surface.
A)I and III
B)I and IV
C)II and III
D)II and IV
E)I,II,and III
I The NaOH wash is thought to generate the Si-OH groups on the silica surface.
II The NaOH wash removes any acidic contaminants from the silica surface.
III The HCl wash is thought to generate the Si-OH groups on the silica surface.
IV The HCl wash removes metal ions from the capillary surface.
A)I and III
B)I and IV
C)II and III
D)II and IV
E)I,II,and III

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
Which is NOT true for apparent,electroosmotic,and electrophoretic mobilities?
A)( app = eo + ep)
B)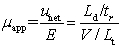
C)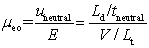
D)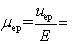
app + eo
E)uapp = ( eo + ep)E
A)( app = eo + ep)
B)
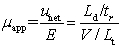
C)
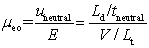
D)
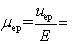
app + eo
E)uapp = ( eo + ep)E

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
The number of plates,N,for a capillary electrophoresis is defined to be 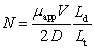 .Which statement is NOT true?
.Which statement is NOT true?
A)As the apparent mobility, app,increases,the number of plates increases.
B)As the total length of the capillary,Lt,increases,the number of plates increases.
C)As the diffusion coefficient,D,increases,the number of plates decreases.
D)As the distance to the detector,Ld,increases,the number of plates increases.
E)As the voltage,V,decreases,the number of plates decreases.
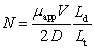 .Which statement is NOT true?
.Which statement is NOT true?A)As the apparent mobility, app,increases,the number of plates increases.
B)As the total length of the capillary,Lt,increases,the number of plates increases.
C)As the diffusion coefficient,D,increases,the number of plates decreases.
D)As the distance to the detector,Ld,increases,the number of plates increases.
E)As the voltage,V,decreases,the number of plates decreases.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
Which statements are true for electroosmotic velocity?
I Electroosmotic velocity,ueo,is calculated experimentally using the retention time of a neutral molecule and the length of the column from the injector to the detector.
II Electroosmotic velocity,ueo,is the product of the electroosmotic mobility, eo,and the electric field,E.
III Electroosmotic velocity,ueo,is inversely proportional to electric field strength,E.
IV Electroosmotic velocity is equal to the electroosmotic flow.
A)II and IV
B)II,III,and IV
C)I,III,and IV
D)I and II
E)I,II,and IV
I Electroosmotic velocity,ueo,is calculated experimentally using the retention time of a neutral molecule and the length of the column from the injector to the detector.
II Electroosmotic velocity,ueo,is the product of the electroosmotic mobility, eo,and the electric field,E.
III Electroosmotic velocity,ueo,is inversely proportional to electric field strength,E.
IV Electroosmotic velocity is equal to the electroosmotic flow.
A)II and IV
B)II,III,and IV
C)I,III,and IV
D)I and II
E)I,II,and IV

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
Under what conditions will anions be detected at the cathode during capillary electrophoresis?
A)( eo > ep)
B)( eo < ep)
C)( eo = ep)
D)( eo + ep > 1)
E)( eo − ep < 1)
A)( eo > ep)
B)( eo < ep)
C)( eo = ep)
D)( eo + ep > 1)
E)( eo − ep < 1)

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
X−,Y−,and Z− are separated with an anion exchange column with a mobile phase containing A−.The strength,which the anions bind to the column,is Y− > X− > Z− > A−.To elute the anions,the concentration of A− is____________________ ,with____________________ eluting first,____________________ eluting second,and____________________ eluting last.
A)decreased;Y−;X−;Z−
B)increased;Z−;X−;Y−
C)decreased;Z−;X−;Y−
D)increased;Y−;X−;Z−
E)increased;X−;Y−;Z−
A)decreased;Y−;X−;Z−
B)increased;Z−;X−;Y−
C)decreased;Z−;X−;Y−
D)increased;Y−;X−;Z−
E)increased;X−;Y−;Z−

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck



