Deck 29: Sample Preparation
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/19
Play
Full screen (f)
Deck 29: Sample Preparation
1
What mass of a solid sample containing 2.5% analyte particles and 97.5% solvent particles must be taken to give a standard deviation of 2% of the number of analyte particles,if the average particle volume is 2.54 nL and the sample has an average density of 1.548 g/mL?
0.3834 g sample
2
Sample preparation of a laboratory sample can involve all of the following,except:
A)laboratory samples that do not dissolve under mild conditions may require acid digestion or fusion.
B)organic material may be destroyed by combustion or wet ashing.
C)solids are typically dried at 110 oC at atmospheric pressure to remove absorbed water prior to analysis.
D)a portion of a course solid laboratory sample should be ground into a fine powder for analysis.
E)temperature-sensitive samples may be stored in an environment to achieve a constant,reproducible moisture level.
A)laboratory samples that do not dissolve under mild conditions may require acid digestion or fusion.
B)organic material may be destroyed by combustion or wet ashing.
C)solids are typically dried at 110 oC at atmospheric pressure to remove absorbed water prior to analysis.
D)a portion of a course solid laboratory sample should be ground into a fine powder for analysis.
E)temperature-sensitive samples may be stored in an environment to achieve a constant,reproducible moisture level.
a portion of a course solid laboratory sample should be ground into a fine powder for analysis.
3
A mixture contains 2.5% KBr and 97.5% inert particles.What is the relative standard deviation for KBr is 50 000 particles are taken?
2.79% for KBr
4
For random errors,the overall variance,  ,is calculated from the analytical variance,
,is calculated from the analytical variance, 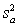 ,and the sampling variance,
,and the sampling variance, 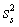 .Which is the correct equation for calculating the overall variance?
.Which is the correct equation for calculating the overall variance?
A)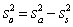
B)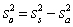
C)
D)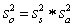
E)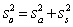
 ,is calculated from the analytical variance,
,is calculated from the analytical variance, 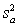 ,and the sampling variance,
,and the sampling variance, 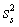 .Which is the correct equation for calculating the overall variance?
.Which is the correct equation for calculating the overall variance?A)
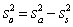
B)
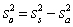
C)

D)
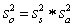
E)
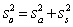

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
5
____________________ dissolves substances that will not dissolve in acid in a hot,molten inorganic____________________.
A)Smelting;solvent
B)Fusion;flux
C)Smelting;flux
D)Fusion;solvent
E)None of these choices is correct.
A)Smelting;solvent
B)Fusion;flux
C)Smelting;flux
D)Fusion;solvent
E)None of these choices is correct.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
6
Sample storage is an important aspect of sample preparation.Which is NOT true for sample storage?
A)Glass should not be used with trace analysis of ions and proteins in solution.
B)Analytes may react with the container.
C)A stored sample may change with time.
D)New plastic containers do not require washing before use.
E)A stored sample may react with air.
A)Glass should not be used with trace analysis of ions and proteins in solution.
B)Analytes may react with the container.
C)A stored sample may change with time.
D)New plastic containers do not require washing before use.
E)A stored sample may react with air.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
7
________________________________________uses a small volume of chromatographic stationary phase or molecularly imprinted polymer to isolate analytes from the sample matrix to simply analysis.
A)Solid-phase extraction
B)Polymer extraction
C)Chromatographic extraction
D)Antigen extraction
E)Solvent-free extraction
A)Solid-phase extraction
B)Polymer extraction
C)Chromatographic extraction
D)Antigen extraction
E)Solvent-free extraction

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
8
Which samplng term is incorrectly defined?
A)laboratory sample - smaller,homogeneous sample taken from the bulk and has the same composition as the bulk
B)lot - the total material from which samples are taken
C)aliquot - small portions of the bulk sample taken for individual analysis
D)sample preparation - the series of steps that convert a representative bulk sample into a form suitable for analysis
E)bulk sample - representative sample taken from the lot for analysis or archiving
A)laboratory sample - smaller,homogeneous sample taken from the bulk and has the same composition as the bulk
B)lot - the total material from which samples are taken
C)aliquot - small portions of the bulk sample taken for individual analysis
D)sample preparation - the series of steps that convert a representative bulk sample into a form suitable for analysis
E)bulk sample - representative sample taken from the lot for analysis or archiving

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
9
All of the following are used to grind solids into a fine powder,except:
A)mortar and pestle.
B)Wig-L-Bug
C)Shatterbox laboratory mill.
D)ball mill.
E)vortexer.
A)mortar and pestle.
B)Wig-L-Bug
C)Shatterbox laboratory mill.
D)ball mill.
E)vortexer.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
10
What mass will give a sampling standard deviation of 3.5% for a sampling constant of 40.9?

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
11
To calculate the number of required replicates for analysis the equation 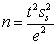 is used.Which statement is NOT true for the equation and calculation?
is used.Which statement is NOT true for the equation and calculation?
A)t = the Student's t value for n − 1.
B)ss is the sampling standard deviation.
C)e is the difference between the sampling standard deviation and the desired sampling standard deviation.
D)The initial calculation assumes an infinite n value and the according Student's t value.
E)The n value from a calculation dictates the Student's t value used in the next calculation.
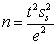 is used.Which statement is NOT true for the equation and calculation?
is used.Which statement is NOT true for the equation and calculation?A)t = the Student's t value for n − 1.
B)ss is the sampling standard deviation.
C)e is the difference between the sampling standard deviation and the desired sampling standard deviation.
D)The initial calculation assumes an infinite n value and the according Student's t value.
E)The n value from a calculation dictates the Student's t value used in the next calculation.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is INCORRECT for decomposition of organic substances?
A)Microwave-induced combustion is a form of dry ashing.
B)An example of wet ashing is microwave digestion with acid in a Teflon bomb.
C)Wet ashing with perchloric aicd is an extremely hazardous procedure and is best avoided.
D)Kjeldahl digestion digests organic material with sulfuric acid for nitrogen analysis.
E)The Carius method of wet ashing is performed with fuming H2SO4 in a sealed,heavy-walled glass tube at 200 oC to 300 oC.
A)Microwave-induced combustion is a form of dry ashing.
B)An example of wet ashing is microwave digestion with acid in a Teflon bomb.
C)Wet ashing with perchloric aicd is an extremely hazardous procedure and is best avoided.
D)Kjeldahl digestion digests organic material with sulfuric acid for nitrogen analysis.
E)The Carius method of wet ashing is performed with fuming H2SO4 in a sealed,heavy-walled glass tube at 200 oC to 300 oC.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
13
Calculate the overall standard deviation if the analytical standard deviation is 6% and the sampling standard deviation is 4%.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
14
A mixture contains 5% NaCl particles and 95% NaBr particles.If 103 particles are taken,what is the relative standard deviation for NaCl and NaBr?
A)13.78% for NaCl 0.73% for NaBr
B)186.2% for NaCl 9.80% for NaBr
C)95.00% for NaCl 5.00% for NaBr
D)54.39% for NaCl 2.86% for NaBr
E)6)89% for NaCl 0.36% for NaBr
A)13.78% for NaCl 0.73% for NaBr
B)186.2% for NaCl 9.80% for NaBr
C)95.00% for NaCl 5.00% for NaBr
D)54.39% for NaCl 2.86% for NaBr
E)6)89% for NaCl 0.36% for NaBr

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
15
All of the following are true for dissolving inorganic materials in acid,except:
A)metals with positive reduction potentials should dissolve in nonoxidizing acids such as HCl,HBr,HF,H3PO4,etc.
B)volatile species formed by protonation of anions such as carbonate will be lost from hot acids in open vessels.
C)volatile metal halides such as SnCl4 and some metal oxides can be lost from hot acids in open vessels.
D)hot hydrofluoric acid dissolves silicates.
E)substances that do not dissolve in nonoxidizing acids may dissolve in oxidizing acids such as HNO3,hot concentrated H2SO4,or hot concentrated HClO4.
A)metals with positive reduction potentials should dissolve in nonoxidizing acids such as HCl,HBr,HF,H3PO4,etc.
B)volatile species formed by protonation of anions such as carbonate will be lost from hot acids in open vessels.
C)volatile metal halides such as SnCl4 and some metal oxides can be lost from hot acids in open vessels.
D)hot hydrofluoric acid dissolves silicates.
E)substances that do not dissolve in nonoxidizing acids may dissolve in oxidizing acids such as HNO3,hot concentrated H2SO4,or hot concentrated HClO4.

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
16
How many replicates must be completed for sampling deviation of 3.5% to achieve a 1% standard deviation?

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
17
A 4.17 g sample has a 4% sampling standard deviation.What mass sample must be taken to decrease the sampling standard deviation to 2%?
A)8)34 g
B)16.68 g
C)10.43 g
D)4)17 g
E)15.64 g
A)8)34 g
B)16.68 g
C)10.43 g
D)4)17 g
E)15.64 g

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
18
____________________ is the procedure in which analyte is chemically modified to make the compound easier to detect or separate.
A)Labeling
B)Derivatization
C)Tagging
D)Marking
E)Modification
A)Labeling
B)Derivatization
C)Tagging
D)Marking
E)Modification

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following are NOT liquid extraction techniques?
I emulsive liquid-liquid microextraction
II dispersive liquid-liquid microextraction
III solid-supported liquid-liquid extraction
IV supercritical fluid extraction
V solid-liquid microextraction
A)I and II
B)III and V
C)I and IV
D)IV only
E)I and III
I emulsive liquid-liquid microextraction
II dispersive liquid-liquid microextraction
III solid-supported liquid-liquid extraction
IV supercritical fluid extraction
V solid-liquid microextraction
A)I and II
B)III and V
C)I and IV
D)IV only
E)I and III

Unlock Deck
Unlock for access to all 19 flashcards in this deck.
Unlock Deck
k this deck



