Deck 8: Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/44
Play
Full screen (f)
Deck 8: Chemical Reactions
1
Consider the following particulate-level representation.The larger spheres represent N and the smaller spheres represent H. 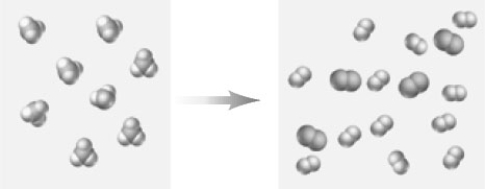 Which of the following correctly characterizes this representation?
Which of the following correctly characterizes this representation?
A)The reactant could be represented as NH3.
B)The products of the reaction are N2 and H2.
C)The equation is "balanced".
D)If the equation for the reaction were written as shown above the coefficient of the nitrogen containing product would be 4.
E)All of these correctly characterize this reaction.
 Which of the following correctly characterizes this representation?
Which of the following correctly characterizes this representation?A)The reactant could be represented as NH3.
B)The products of the reaction are N2 and H2.
C)The equation is "balanced".
D)If the equation for the reaction were written as shown above the coefficient of the nitrogen containing product would be 4.
E)All of these correctly characterize this reaction.
All of these correctly characterize this reaction.
2
Balance the equation C8H18(l)+ ___ O2(g)→ ___ CO2(g)+ ___ H2O(l).
What is the coefficient for O2(g)?
A)16
B)9
C)25
D)12
E)50
What is the coefficient for O2(g)?
A)16
B)9
C)25
D)12
E)50
25
3
Which of the following generally indicates the possibility of a chemical change?
i.Color change
ii.Formation of a solid
iii.Formation of a gas
iv.Absorption or release of heat energy
v.Emission of light energy
A)All
B)All but i
C)All but ii
D)All but iii
E)All but v
i.Color change
ii.Formation of a solid
iii.Formation of a gas
iv.Absorption or release of heat energy
v.Emission of light energy
A)All
B)All but i
C)All but ii
D)All but iii
E)All but v
All
4
Calcium combines with bromine to make calcium bromide.Write the balanced chemical equation for this reaction.What is the coefficient for bromine?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
5
What is the coefficient for H2 when the equation ___ Ba + ___ H3AsO4 → ___ H2 + ___ Ba3(AsO4)2 is properly balanced?
A)1
B)3
C)5
D)2
E)6
A)1
B)3
C)5
D)2
E)6

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
6
A chemical equation may sometimes be balanced with fractional coefficients.This is not appropriate when utilizing what interpretation of the reaction?
A)Macroscopic
B)Molar
C)Particulate
D)Both macroscopic and molar
E)Both molar and particulate
A)Macroscopic
B)Molar
C)Particulate
D)Both macroscopic and molar
E)Both molar and particulate

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
7
12.09 g of A2 reacts with 96.00 g of B2 to form 108.09 g of A2B.What is the ratio of the molar mass of A to the molar mass of B?
A)1:8
B)8:1
C)1:4
D)16:1
E)1:16
A)1:8
B)8:1
C)1:4
D)16:1
E)1:16

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
8
Consider the hypothetical chemical reaction represented by the equation A + 2 B → 3 C + D Which of the following interpretations of this equation is not correct?
A)1 kg of A reacts with 2 kg of B to form 3 kg of C and 1 kg of D
B)1 particle of A reacts with 2 particles of B to form 3 particles of C and 1 particle of D
C)1 dozen particles of A reacts with 2 dozen particles of B to form 3 dozen particles of C and 1 dozen particles of D
D)1 mole of particles of A reacts with 2 moles of particles of B to form 3 moles of particles of C and 1 mole of particles of D
E)1 kilomole of particles of A reacts with 2 kilomoles of particles of B to form 3 kilomoles of particles of C and 1 kilomole of particles of D
A)1 kg of A reacts with 2 kg of B to form 3 kg of C and 1 kg of D
B)1 particle of A reacts with 2 particles of B to form 3 particles of C and 1 particle of D
C)1 dozen particles of A reacts with 2 dozen particles of B to form 3 dozen particles of C and 1 dozen particles of D
D)1 mole of particles of A reacts with 2 moles of particles of B to form 3 moles of particles of C and 1 mole of particles of D
E)1 kilomole of particles of A reacts with 2 kilomoles of particles of B to form 3 kilomoles of particles of C and 1 kilomole of particles of D

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
9
Consider the hypothetical chemical reaction represented by the equation 3 A + 2 B → A3B2 Which of the following is a correct interpretation of this equation?
i.3 grams of A react with 2 grams of B to form 1 gram of A3B2
ii.3 atoms of A react with 2 atoms of B to form 1 molecule of A3B2
iii.3 moles of A react with 2 moles of B to form 1 mole of A3B2
A)i only
B)ii only
C)iii only
D)ii and iii
E)i, ii, and iii
i.3 grams of A react with 2 grams of B to form 1 gram of A3B2
ii.3 atoms of A react with 2 atoms of B to form 1 molecule of A3B2
iii.3 moles of A react with 2 moles of B to form 1 mole of A3B2
A)i only
B)ii only
C)iii only
D)ii and iii
E)i, ii, and iii

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
10
What is the coefficient for carbon dioxide when the equation ___ C2H6 + ___ O2 → ___ CO2 + ___ H2O is properly balanced?
A)7
B)6
C)5
D)4
E)2
A)7
B)6
C)5
D)4
E)2

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
11
When atomic phosphorous (P)and oxygen are directly combined,P4O10 is produced.Write the balanced chemical equation for this reaction.What is the sum of the coefficients?
A)3 or less
B)5
C)7
D)9
E)10 or more
A)3 or less
B)5
C)7
D)9
E)10 or more

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
12
Calcium reacts with the oxygen in the air,forming calcium oxide.Write the balanced chemical equation for this reaction.What is the sum of the coefficients?
A)3 or less
B)4
C)5
D)6
E)7 or more
A)3 or less
B)4
C)5
D)6
E)7 or more

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
13
When potassium contacts fluorine gas,potassium fluoride is produced.Write the balanced chemical equation for this reaction.What is the coefficient for potassium fluoride?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
14
A pot of room temperature water is placed on a stovetop burner.After a couple of minutes,small,odorless,colorless bubbles form and bubble out of the liquid.After a few more minutes,similar,larger bubbles form and bubble out at a faster rate.Which of the following is the best explanation of the observations?
A)No change occurs because the bubbles contain no matter.
B)The smaller bubbles are a result of a physical change, and the larger bubbles are the result of a chemical change.
C)The smaller bubbles are the result of a chemical change, and the larger bubbles are the result of a physical change.
D)Both the smaller and larger bubbles are the result of a physical change.
E)Both the smaller and larger bubbles are the result of a chemical change.
A)No change occurs because the bubbles contain no matter.
B)The smaller bubbles are a result of a physical change, and the larger bubbles are the result of a chemical change.
C)The smaller bubbles are the result of a chemical change, and the larger bubbles are the result of a physical change.
D)Both the smaller and larger bubbles are the result of a physical change.
E)Both the smaller and larger bubbles are the result of a chemical change.

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
15
When the equation ___ PCl5 + ___ H2O → ___ H3PO4 + ___ HCl is properly balanced,what is the sum of the coefficients?
A)8 or less
B)9
C)10
D)11
E)12 or more
A)8 or less
B)9
C)10
D)11
E)12 or more

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
16
Consider the following particulate-level representation of a chemical equation:  Assume that all spheres represent atoms of A.Which of the following is a correctly balanced equation for this reaction?
Assume that all spheres represent atoms of A.Which of the following is a correctly balanced equation for this reaction?
A)A → 2 A
B)A2 → 2 A
C)2 A → 2 A
D)A → 1/2 A + 1/2 A
E)A(s) → A(g)
 Assume that all spheres represent atoms of A.Which of the following is a correctly balanced equation for this reaction?
Assume that all spheres represent atoms of A.Which of the following is a correctly balanced equation for this reaction?A)A → 2 A
B)A2 → 2 A
C)2 A → 2 A
D)A → 1/2 A + 1/2 A
E)A(s) → A(g)

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
17
Ammonia is formed from its elements.Write the balanced chemical equation for this reaction.What is the sum of the coefficients?
A)3 or less
B)4
C)5
D)6
E)7 or more
A)3 or less
B)4
C)5
D)6
E)7 or more

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
18
When the equation ___ Ca(OH)2 + ___ H3PO4 → ___ Ca3(PO4)2 + ___ H2O is properly balanced,what is the sum of the coefficients?
A)8 or less
B)9
C)10
D)11
E)12 or more
A)8 or less
B)9
C)10
D)11
E)12 or more

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
19
What is the coefficient for Zn when the equation ___ Zn + ___ H3PO4 → ___ H2 + ___ Zn3(PO4)2 is properly balanced?
A)1
B)2
C)3
D)4
E)6
A)1
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
20
The following image shows the result of mixing an aqueous solution of silver nitrate with an aqueous solution of sodium chloride.  The products of the reaction are a solid and a solution.The physical states of the reaction in the equation should be specified using which set of state symbols,respectively?
The products of the reaction are a solid and a solution.The physical states of the reaction in the equation should be specified using which set of state symbols,respectively?
A)(l), (l), (s), (l)
B)(aq), (aq), (s), (aq)
C)(aq), (aq), (s), (l)
D)(l), (l), (s), (aq)
E)(aq), (aq), (p), (aq)
 The products of the reaction are a solid and a solution.The physical states of the reaction in the equation should be specified using which set of state symbols,respectively?
The products of the reaction are a solid and a solution.The physical states of the reaction in the equation should be specified using which set of state symbols,respectively?A)(l), (l), (s), (l)
B)(aq), (aq), (s), (aq)
C)(aq), (aq), (s), (l)
D)(l), (l), (s), (aq)
E)(aq), (aq), (p), (aq)

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
21
Solid magnesium hydroxide is added to hydrochloric acid.Write the balanced chemical equation for this reaction.What is the sum of the coefficients?
A)4
B)5
C)6
D)7
E)8 or more
A)4
B)5
C)6
D)7
E)8 or more

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
22
Bromine is added to a solution of sodium iodide.Write the balanced chemical equation for this reaction.What is the sum of the coefficients?
A)4 or less
B)5
C)6
D)7
E)8 or more
A)4 or less
B)5
C)6
D)7
E)8 or more

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
23
A precipitate forms when solutions of magnesium bromide and sodium hydroxide are combined.Write the balanced chemical equation for this reaction.What is the coefficient for magnesium bromide?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
24
Pure hydrogen iodide decomposes spontaneously to its elements.Write the balanced chemical equation for this reaction.What is the sum of the coefficients?
A)5
B)3
C)7
D)4
E)6
A)5
B)3
C)7
D)4
E)6

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
25
Lithium combines with oxygen to form lithium oxide.Write the balanced chemical equation for this reaction.What is the coefficient for lithium?
A)4
B)3
C)2
D)1
E)none of the above
A)4
B)3
C)2
D)1
E)none of the above

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
26
Examine the following two beakers.  When the equation for this reaction is balanced,what should the coefficient of Al?
When the equation for this reaction is balanced,what should the coefficient of Al?
A)1
B)2
C)3
D)4
E)6
 When the equation for this reaction is balanced,what should the coefficient of Al?
When the equation for this reaction is balanced,what should the coefficient of Al?A)1
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
27
Molten sodium chloride is decomposed into its elements by electrolysis.Write the balanced chemical equation for this reaction.What is the sum of the coefficients?
A)3 or less
B)4
C)5
D)6
E)7 or more
A)3 or less
B)4
C)5
D)6
E)7 or more

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
28
Nitric acid reacts with a solution of potassium hydroxide.Write the balanced chemical equation for this reaction.What is the sum of the coefficients?
A)10
B)8
C)6
D)4
E)none of the above
A)10
B)8
C)6
D)4
E)none of the above

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
29
Calcium nitrate and potassium fluoride solutions react to form a precipitate.How should this reaction be classified?
A)combination
B)decomposition
C)double-replacement
D)single-replacement
A)combination
B)decomposition
C)double-replacement
D)single-replacement

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the following particulate-level representation of a reaction. 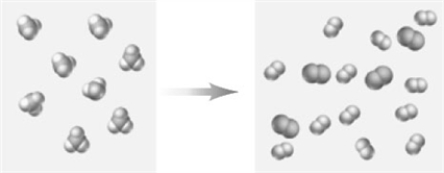 How would this reaction be classified.
How would this reaction be classified.
A)combination
B)decomposition
C)single-replacement
D)double-replacement
 How would this reaction be classified.
How would this reaction be classified.A)combination
B)decomposition
C)single-replacement
D)double-replacement

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
31
Magnesium is placed into sulfuric acid.Write the balanced chemical equation for this reaction.What is the sum of the coefficients?
A)4 or less
B)5
C)6
D)7
E)8 or more
A)4 or less
B)5
C)6
D)7
E)8 or more

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
32
A precipitate forms when magnesium chloride and sodium fluoride solutions are combined.Write the balanced chemical equation for this reaction.What is the sum of the coefficients?
A)6
B)5
C)4
D)9
E)none of the above
A)6
B)5
C)4
D)9
E)none of the above

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
33
Calcium reacts with hydrobromic acid.Write the balanced chemical equation for this reaction.What is the sum of the coefficients?
A)4 or less
B)5
C)6
D)7
E)8 or more
A)4 or less
B)5
C)6
D)7
E)8 or more

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
34
When sodium chloride and silver nitrate solutions are combined,the reaction shown below occurs.  Write the balanced chemical equation for this reaction.What is the coefficient for silver nitrate?
Write the balanced chemical equation for this reaction.What is the coefficient for silver nitrate?
A)5
B)4
C)3
D)2
E)1
 Write the balanced chemical equation for this reaction.What is the coefficient for silver nitrate?
Write the balanced chemical equation for this reaction.What is the coefficient for silver nitrate?A)5
B)4
C)3
D)2
E)1

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
35
Barium peroxide,BaO2,breaks down into barium oxide and oxygen.Write the balanced chemical equation for this reaction.What is the coefficient for barium oxide?
A)3
B)1
C)5
D)4
E)2
A)3
B)1
C)5
D)4
E)2

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
36
A piece of potassium is placed in a phosphoric acid solution.Write the balanced chemical equation for this reaction.What is the coefficient for potassium?
A)1
B)2
C)3
D)4
E)none of the above
A)1
B)2
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
37
Solid aluminum is dropped into a solution of sulfuric acid.Write the balanced chemical equation for this reaction.What is the coefficient for sulfuric acid?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
38
Consider the following particulate-level representation of a reaction 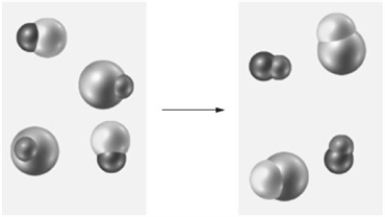 How is the reaction classified?
How is the reaction classified?
A)combination
B)decomposition
C)single-replacement
D)double-replacement
 How is the reaction classified?
How is the reaction classified?A)combination
B)decomposition
C)single-replacement
D)double-replacement

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
39
Lead (II)carbonate decomposes to lead (II)oxide and carbon dioxide.Write the balanced chemical equation for this reaction.What is the coefficient for lead (II)carbonate?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
40
The combination of copper (II)nitrate and ammonium sulfide solutions yields a precipitate.Write the balanced chemical equation for this reaction.What is the coefficient for copper (II)nitrate?
A)3
B)1
C)5
D)2
E)4
A)3
B)1
C)5
D)2
E)4

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
41
Phosphoric acid reacts with sodium hydroxide.Write the balanced chemical equation for this reaction.What is the coefficient for sodium hydroxide?
A)3
B)6
C)2
D)1
E)4
A)3
B)6
C)2
D)1
E)4

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
42
Sulfuric acid neutralizes a barium hydroxide solution.Write the balanced chemical equation for this reaction.What is the sum of the coefficients?
A)4
B)5
C)6
D)7
E)8 or more
A)4
B)5
C)6
D)7
E)8 or more

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
43
Calcium hydroxide is added to perchloric acid.Write the balanced chemical equation for this reaction.What is the coefficient for perchloric acid?
A)5
B)4
C)3
D)2
E)1
A)5
B)4
C)3
D)2
E)1

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck
44
Aluminum hydroxide is added to nitric acid.Write the balanced chemical equation for this reaction.What is the coefficient for aluminum hydroxide?
A)6
B)4
C)3
D)2
E)1
A)6
B)4
C)3
D)2
E)1

Unlock Deck
Unlock for access to all 44 flashcards in this deck.
Unlock Deck
k this deck



