Deck 16: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/38
Play
Full screen (f)
Deck 16: Nuclear Chemistry
1
Uranium-235 decays by alpha emission.What isotope is also produced by this transformation?
A)Pa-234
B)Th-237
C)Pu-237
D)Th-231
E)Pu-239
A)Pa-234
B)Th-237
C)Pu-237
D)Th-231
E)Pu-239
Th-231
2
When atoms of beryllium−9 are bombarded with alpha particles, neutrons are produced.What new isotope is also formed? 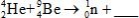
A)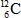
B)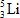
C)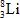
D)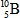
E)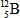
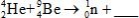
A)
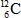
B)
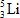
C)
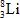
D)
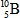
E)
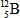
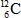
3
In the equation below, what particle or type of radiation needs to be included to balance the equation? 208Po → ? + 208At
A)Gamma particle
B)Alpha particle
C)Proton
D)Beta particle
E)Positron
A)Gamma particle
B)Alpha particle
C)Proton
D)Beta particle
E)Positron
Beta particle
4
Which is an incorrect representation of the indicated particle or nucleus?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
5
What other particle is emitted when a neutron is converted to a proton in a nucleus?
A)Gamma particle
B)Alpha particle
C)Positron
D)Beta particle
E)None of the answers is correct.
A)Gamma particle
B)Alpha particle
C)Positron
D)Beta particle
E)None of the answers is correct.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
6
When atoms of aluminum-27 are bombarded with alpha particles, a neutron and an element are produced.Which particular isotope of this element is formed? 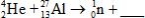
A)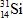
B)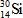
C)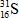
D)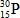
E)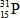
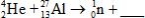
A)
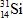
B)
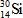
C)
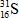
D)
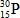
E)
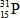

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
7
What fraction of radioactive atoms remains in a sample after six half-lives?
A)zero
B)1/6
C)1/16
D)1/32
E)1/64
A)zero
B)1/6
C)1/16
D)1/32
E)1/64

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
8
In the equation below, what particle or type of radiation needs to be included to balance the equation? 220Rn → ? + alpha particle
A)Ra-224
B)Rn-224
C)Rn-216
D)At-220
E)Po-216
A)Ra-224
B)Rn-224
C)Rn-216
D)At-220
E)Po-216

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
9
Beta particles are identical to
A)protons.
B)helium atoms.
C)hydrogen atoms.
D)helium nuclei.
E)electrons.
A)protons.
B)helium atoms.
C)hydrogen atoms.
D)helium nuclei.
E)electrons.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
10
What is the missing symbol in this plutonium fission reaction? 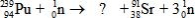
A)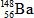
B)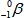
C)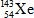
D)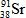
E)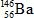
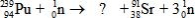
A)
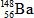
B)
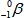
C)
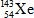
D)
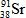
E)
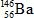

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
11
What is the name for spontaneous emission of particles or electromagnetic radiation by certain nuclei?
A)Protons
B)Isotopes
C)Radioactivity
D)Neutrons
E)Electrons
A)Protons
B)Isotopes
C)Radioactivity
D)Neutrons
E)Electrons

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
12
Alpha particles are identical to
A)protons.
B)helium atoms.
C)hydrogen atoms.
D)helium nuclei.
E)electrons.
A)protons.
B)helium atoms.
C)hydrogen atoms.
D)helium nuclei.
E)electrons.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
13
The only stable isotope of aluminum is aluminum-27.What type of radioactive decay should be expected from 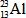 ?
?
A)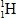
B)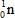
C)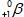
D)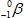
E)
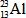 ?
?A)
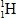
B)
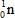
C)
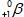
D)
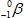
E)


Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
14
Iodine-131, t1/2 = 8.0 days, is used in the diagnosis and treatment of thyroid gland diseases.If a laboratory sample of iodine-131 initially emits 9.95 × 1018 β particles per day, how long will it take for the activity to drop to 6.22 × 1017 β particles per day?
A)2.0 days
B)16 days
C)32 days
D)128 days
E)None of the answers is correct.
A)2.0 days
B)16 days
C)32 days
D)128 days
E)None of the answers is correct.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
15
Cesium-134 is a β emitter with a half-life of 2.0 years.How much of a 2.50-g sample of cesium-134 will remain after 10 years?
A)0.0024 g
B)0.078 g
C)0.25 g
D)0.50 g
E)80.0 g
A)0.0024 g
B)0.078 g
C)0.25 g
D)0.50 g
E)80.0 g

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
16
What is the name given to the nuclear process that results from the bombardment of nuclei by neutrons, protons, or other nuclei?
A)Nuclear transmutation
B)Protonation
C)Nucleation
D)Nuclear condensation
E)Radioactivity
A)Nuclear transmutation
B)Protonation
C)Nucleation
D)Nuclear condensation
E)Radioactivity

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
17
Which equation correctly represents positron decay of 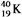 ?
?
A)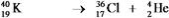
B)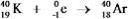
C)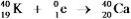
D)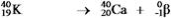
E)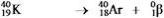
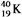 ?
?A)
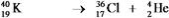
B)
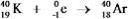
C)
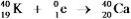
D)
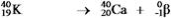
E)
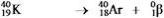

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
18
In the equation below, what particle or type of radiation needs to be included to balance the equation? 234Th → ? + beta particle
A)Ac-234
B)Pa-234
C)Ac-235
D)Pa-235
E)Ac-233
A)Ac-234
B)Pa-234
C)Ac-235
D)Pa-235
E)Ac-233

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
19
As a result of beta decay, the product nucleus is
A)one atomic number lower than the original element.
B)two atomic numbers higher than the original element.
C)one atomic number higher than the original element.
D)two atomic numbers lower than the original element.
E)four atomic numbers lower than the original element.
A)one atomic number lower than the original element.
B)two atomic numbers higher than the original element.
C)one atomic number higher than the original element.
D)two atomic numbers lower than the original element.
E)four atomic numbers lower than the original element.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
20
In the equation below, what particle or type of radiation needs to be included to balance the equation? 208Pb → ? + 204Hg
A)Gamma particle
B)Alpha particle
C)Proton
D)Beta particle
E)Positron
A)Gamma particle
B)Alpha particle
C)Proton
D)Beta particle
E)Positron

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is used to image the brain?
A)(18O)
B)(131I)
C)(123I)
D)(24Na)
E)(99Tc)
A)(18O)
B)(131I)
C)(123I)
D)(24Na)
E)(99Tc)

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
22
In the following reaction, identify X. 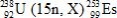
A)7β
B)3α
C)4n
D)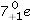
E)15p
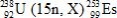
A)7β
B)3α
C)4n
D)
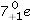
E)15p

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
23
The energy released by the sun is the result of
A)natural radioactivity.
B)nuclear fusion.
C)combustion of hydrogen.
D)photosynthesis.
E)nuclear fission.
A)natural radioactivity.
B)nuclear fusion.
C)combustion of hydrogen.
D)photosynthesis.
E)nuclear fission.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
24
Cobalt-60 is a beta emitter with a half-life of 5.3 years.Approximately what fraction of cobalt-60 atoms will remain in a particular sample after 26.5 years?
A)1/5
B)1/16
C)1/26
D)1/32
E)1/64
A)1/5
B)1/16
C)1/26
D)1/32
E)1/64

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
25
Identify the missing species in the following nuclear transmutation. 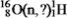
A)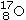
B)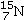
C)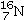
D)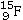
E)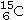
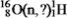
A)
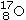
B)
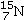
C)
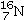
D)
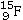
E)
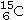

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
26
What is the nuclear process called where small nuclei are combined into larger ones?
A)Photonuclear reactions
B)Nuclear fission
C)Thermal conductivity
D)Nuclear combination
E)Nuclear fusion
A)Photonuclear reactions
B)Nuclear fission
C)Thermal conductivity
D)Nuclear combination
E)Nuclear fusion

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
27
The half-life of 14C is 5730 yr.Assuming some charcoal from a campfire 29,000 years old was found, what fraction of the original C-14 would remain today?
A)3.0 × 10−2
B)0.20
C)3.5
D)0.33
E)0.29
A)3.0 × 10−2
B)0.20
C)3.5
D)0.33
E)0.29

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
28
In the following reaction, identify X. 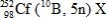
A)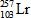
B)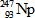
C)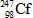
D)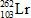
E)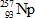
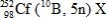
A)
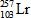
B)
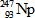
C)
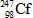
D)
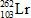
E)
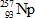

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is used to image the liver?
A)(18O)
B)(131I)
C)(123I)
D)(24Na)
E)(99Tc)
A)(18O)
B)(131I)
C)(123I)
D)(24Na)
E)(99Tc)

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
30
Identify the missing species in the following nuclear transmutation.U-238 + ? → 1 neutron + Fm-249
A)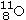
B)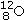
C)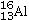
D)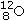
E)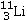
A)
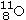
B)
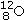
C)
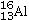
D)
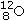
E)
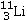

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
31
Identify the missing species in the following nuclear transmutation. 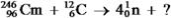
A)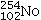
B)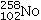
C)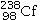
D)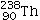
E)None of the answers is correct.
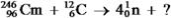
A)
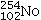
B)
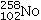
C)
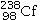
D)
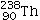
E)None of the answers is correct.

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
32
Estimate the age of a bottled wine that has a tritium, 3H, content 60% that of freshly bottled wine.Tritium decays by beta decay and has a half-life of 12.3 yr. 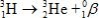
A)0.029 yr
B)7.4 yr
C)9.1 yr
D)16 yr
E)35 yr
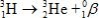
A)0.029 yr
B)7.4 yr
C)9.1 yr
D)16 yr
E)35 yr

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is used to image the heart?
A)(18O)
B)(131I)
C)(123I)
D)(24Na)
E)(99Tc)
A)(18O)
B)(131I)
C)(123I)
D)(24Na)
E)(99Tc)

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is used to test the activity of the thyroid?
A)(18O)
B)(131I)
C)(123I)
D)(24Na)
E)(99Tc)
A)(18O)
B)(131I)
C)(123I)
D)(24Na)
E)(99Tc)

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
35
Which type of nuclear process requires an extremely high temperature (millions of degrees)?
A)Beta decay
B)Fission reaction
C)Fusion reaction
D)Alpha decay
E)Positron emission
A)Beta decay
B)Fission reaction
C)Fusion reaction
D)Alpha decay
E)Positron emission

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
36
Charcoal found under a stone at Stonehenge, England, has a carbon-14 activity that is 0.60 that of new wood.How old is the charcoal? (The half-life of carbon-14 is 5730 years.)
A)Less than 5730 yr
B)Between 5730 and 11,460 yr
C)Between 11,460 and 17,190 yr
D)More than 17,190 yr
A)Less than 5730 yr
B)Between 5730 and 11,460 yr
C)Between 11,460 and 17,190 yr
D)More than 17,190 yr

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
37
Identify the missing species in the following nuclear transmutation. 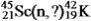
A)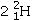
B)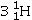
C)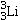
D)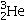
E)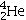
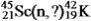
A)
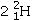
B)
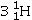
C)
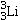
D)
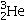
E)
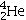

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck
38
What is the nuclear process called in which a heavy nuclear of mass number greater than 200 divides to form smaller nuclei of intermediate mass and one or more neutron(s)?
A)Photonuclear reactions
B)Nuclear fission
C)Thermal conductivity
D)Nuclear combination
E)Nuclear fusion
A)Photonuclear reactions
B)Nuclear fission
C)Thermal conductivity
D)Nuclear combination
E)Nuclear fusion

Unlock Deck
Unlock for access to all 38 flashcards in this deck.
Unlock Deck
k this deck



