Deck 8: Atoms and Periodic Properties
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/30
Play
Full screen (f)
Deck 8: Atoms and Periodic Properties
1
Atoms in an incandescent gas
A)emit all frequencies of light in a continuous spectrum.
B)emit different frequencies of light depending on its temperature.
C)emit characteristic frequencies of light.
D)absorb, rather than emit light.
A)emit all frequencies of light in a continuous spectrum.
B)emit different frequencies of light depending on its temperature.
C)emit characteristic frequencies of light.
D)absorb, rather than emit light.
C
2
Heisenberg's uncertainty principle says that you cannot know the momentum or the position of an electron exactly.
False
3
An electron moves from one orbital to another only when it absorbs or emits energy.
True
4
Einstein proposed that electrons on the surface of a metal gradually absorb energy from photons until they have enough energy to leave the surface.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
5
Protons and neutrons have about the same mass.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
6
J.J.Thomson reasoned that cathode rays were really charged particles because
A)a magnet deflected cathode rays.
B)cathode rays formed only when the air was pumped out of a discharge tube.
C)the properties of the cathode rays depended on the cathode material.
D)the cathode rays were attracted to the anode.
A)a magnet deflected cathode rays.
B)cathode rays formed only when the air was pumped out of a discharge tube.
C)the properties of the cathode rays depended on the cathode material.
D)the cathode rays were attracted to the anode.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
7
Protons are so much more massive than electrons that you can neglect the mass of electrons when determining the mass of an atom.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
8
The atomic number of an element is the number of
A)protons.
B)protons and neutrons.
C)protons and electrons.
D)all the particles in the atom.
A)protons.
B)protons and neutrons.
C)protons and electrons.
D)all the particles in the atom.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
9
Neutral atoms of a given element all have the same
A)number of protons.
B)atomic number.
C)number of electrons.
D)All of the above.
A)number of protons.
B)atomic number.
C)number of electrons.
D)All of the above.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
10
Robert Millikan discovered that
A)the charge to mass ratio of electrons was constant.
B)the electron carries the smallest unit of electrical charge.
C)the oil droplets all carried the same amount of charge.
D)the electrons contained most of the mass of an atom.
A)the charge to mass ratio of electrons was constant.
B)the electron carries the smallest unit of electrical charge.
C)the oil droplets all carried the same amount of charge.
D)the electrons contained most of the mass of an atom.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
11
Millikan found that all of the oil droplets in his apparatus carried a charge that was an integer multiple of one particular value.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
12
The atomic number of an element is the total number of protons and neutrons in the nucleus.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
13
J.J.Thomson discovered that cathode rays were really a stream of electrons.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
14
Elements in the same row of the periodic table exhibit similar chemical properties.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
15
Niels Bohr's model of the hydrogen atom
A)was controversial because it contradicted accepted principles of physics.
B)held that electrons existed in allowed orbits and nowhere else.
C)accounted for the observed spectrum in hydrogen.
D)All of the above.
A)was controversial because it contradicted accepted principles of physics.
B)held that electrons existed in allowed orbits and nowhere else.
C)accounted for the observed spectrum in hydrogen.
D)All of the above.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
16
When Rutherford found that some of the alpha particles fired at the gold foil were widely deflected, he concluded that
A)gold was an element, not a compound as previously believed.
B)atoms are solid, with spaces between them.
C)atoms are electrically neutral.
D)the positive charge in an atom is concentrated in a tiny nucleus.
A)gold was an element, not a compound as previously believed.
B)atoms are solid, with spaces between them.
C)atoms are electrically neutral.
D)the positive charge in an atom is concentrated in a tiny nucleus.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
17
The word halogen comes from the Greek, meaning salt-former.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
18
Rutherford concluded from his calculations that the volume of an atom
A)is filled with protons, neutrons, and electrons.
B)is mostly protons, with electrons revolving around the outside.
C)is filled with electrons.
D)is mostly empty space.
A)is filled with protons, neutrons, and electrons.
B)is mostly protons, with electrons revolving around the outside.
C)is filled with electrons.
D)is mostly empty space.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
19
John Dalton reasoned that atoms exist from the evidence that
A)there are numerous, tiny empty spaces in matter.
B)elements always combined in certain fixed ratios.
C)elements could not be broken down into simpler substances.
D)matter was homogeneous.
A)there are numerous, tiny empty spaces in matter.
B)elements always combined in certain fixed ratios.
C)elements could not be broken down into simpler substances.
D)matter was homogeneous.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
20
The fact that wavelengths of the four lines in the Balmer series fit a regular pattern was evidence supporting the idea that
A)electrons could exist in only four energy states in a hydrogen atom.
B)there must be four electrons in each hydrogen atom.
C)electrons could only gain or lose specific amounts of energy in hydrogen atoms.
D)electrons were continuously losing energy.
A)electrons could exist in only four energy states in a hydrogen atom.
B)there must be four electrons in each hydrogen atom.
C)electrons could only gain or lose specific amounts of energy in hydrogen atoms.
D)electrons were continuously losing energy.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
21
Photons of which of the following colors of light possess the greatest amount of energy?
A)blue
B)green
C)yellow
D)red
A)blue
B)green
C)yellow
D)red

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
22
According to the quantum mechanical model, electrons exist in
A)circular orbits.
B)elliptical orbits.
C)orbitals.
D)wavy orbits.
A)circular orbits.
B)elliptical orbits.
C)orbitals.
D)wavy orbits.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
23
Isotopes of an element are atoms that have
A)the same number of protons, but a different number of electron.
B)the same number of neutrons, but a different number of protons.
C)the same number of protons, but a different number of neutrons.
D)equal numbers of protons and neutrons.
A)the same number of protons, but a different number of electron.
B)the same number of neutrons, but a different number of protons.
C)the same number of protons, but a different number of neutrons.
D)equal numbers of protons and neutrons.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
24
In the notation "2p4", the 4 refers to the
A)energy level of the electron.
B)number of p orbitals in the 2nd energy level.
C)number of electrons in the 2p sublevel.
D)number of electrons in a single 2p orbital.
A)energy level of the electron.
B)number of p orbitals in the 2nd energy level.
C)number of electrons in the 2p sublevel.
D)number of electrons in a single 2p orbital.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
25
The proposal that matter, like light, exhibits wave-like behavior was
A)verified in diffraction experiments with a beam of electrons.
B)never tested since it was known to be impossible.
C)shown to be theoretically possible, but never verified by experiment.
D)verified by physical measurements of a moving baseball.
A)verified in diffraction experiments with a beam of electrons.
B)never tested since it was known to be impossible.
C)shown to be theoretically possible, but never verified by experiment.
D)verified by physical measurements of a moving baseball.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
26
The energy of a photon of light emitted by an electron equals the
A)energy of the level it currently occupies.
B)energy of the level it just left.
C)energy of the ground state of the atom.
D)difference in energy between two levels.
A)energy of the level it currently occupies.
B)energy of the level it just left.
C)energy of the ground state of the atom.
D)difference in energy between two levels.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
27
Identify the number of protons, neutrons, and electrons in an atom of 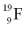 .
.
A)9 protons, 10 neutrons, and 9 electrons
B)9 protons, 10 neutrons, and 10 electrons
C)9 protons, 19 neutrons, and 9 electrons
D)10 protons, 9 neutrons, and 10 electrons
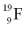 .
.A)9 protons, 10 neutrons, and 9 electrons
B)9 protons, 10 neutrons, and 10 electrons
C)9 protons, 19 neutrons, and 9 electrons
D)10 protons, 9 neutrons, and 10 electrons

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
28
The quantum mechanical model of the atom differs from the Bohr model in that it
A)considers the electron as a particle.
B)considers the electron as a wave.
C)predicts the specific location of the electron in an atom.
D)states that electrons can only exist at specific distances from the nucleus.
A)considers the electron as a particle.
B)considers the electron as a wave.
C)predicts the specific location of the electron in an atom.
D)states that electrons can only exist at specific distances from the nucleus.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
29
The maximum number of electrons that can have the principal quantum number 2 in a given atom is
A)2.
B)4.
C)8.
D)...it varies from atom to atom.
A)2.
B)4.
C)8.
D)...it varies from atom to atom.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
30
In the Bohr model of the atom, the energy state of an electron could be described with one number.The quantum mechanical model of the atom requires how many numbers to do the same?
A)4
B)2
C)1
D)It varies from atom to atom.
A)4
B)2
C)1
D)It varies from atom to atom.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck



