Deck 22: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/85
Play
Full screen (f)
Deck 22: Nuclear Chemistry
1
If the mass of one neutron is 1.00866 amu,the mass of one proton is 1.00728 amu,and the mass of 14C nucleus is 13.99995 amu,calculate the binding energy for the 14C nucleus.
A)9)92 × 109 kJ/mol
B)1)02 × 1010 kJ/mol
C)5)54 × 1011 kJ/mol
D)5)95 × 1011 kJ/mol
A)9)92 × 109 kJ/mol
B)1)02 × 1010 kJ/mol
C)5)54 × 1011 kJ/mol
D)5)95 × 1011 kJ/mol
1)02 × 1010 kJ/mol
2
Which of the following isotopes is the least stable?
A)(7Be,5.37 MeV/nucleon)
B)(23Na,8.11 MeV/nucleon)
C)(94Zr,8.67 MeV/nucleon)
D)(240Pu,7.26 MeV/nucleon)
A)(7Be,5.37 MeV/nucleon)
B)(23Na,8.11 MeV/nucleon)
C)(94Zr,8.67 MeV/nucleon)
D)(240Pu,7.26 MeV/nucleon)
(7Be,5.37 MeV/nucleon)
3
Which of the following is not an advantage of fusion reactions over fission reactions for power generation?
A)cheap and plentiful fuel
B)ease of reaction initiation
C)Fusion products are non-polluting.
D)Fusion products are nonradioactive.
A)cheap and plentiful fuel
B)ease of reaction initiation
C)Fusion products are non-polluting.
D)Fusion products are nonradioactive.
ease of reaction initiation
4
The total binding energies for 3He,4He,and 6He are 7.72 MeV,28.29 MeV,and 29.26 MeV respectively.Arrange the 3 isotopes in increasing order of binding energy per nucleon.
A)(3He < 4He < 6He)
B)(6He < 4He < 3He)
C)(4He < 3He < 6He)
D)(3He < 6He < 4He)
A)(3He < 4He < 6He)
B)(6He < 4He < 3He)
C)(4He < 3He < 6He)
D)(3He < 6He < 4He)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following isotopes is the most stable?
A)(6He,4.89 MeV/nucleon)
B)(20Ne,8.03 MeV/nucleon)
C)(28Si,8.44 MeV/nucleon)
D)(232Th,7.62 MeV/nucleon)
A)(6He,4.89 MeV/nucleon)
B)(20Ne,8.03 MeV/nucleon)
C)(28Si,8.44 MeV/nucleon)
D)(232Th,7.62 MeV/nucleon)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
6
Mass defect is the mass lost when
A)an atom is formed from individual protons,neutrons,and electrons.
B)a nucleus is formed from individual protons and neutrons.
C)electrons are removed from an atom.
D)electrons are added to an atom.
A)an atom is formed from individual protons,neutrons,and electrons.
B)a nucleus is formed from individual protons and neutrons.
C)electrons are removed from an atom.
D)electrons are added to an atom.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
7
How much energy is released for alpha decay of 236U? The masses of 4He,232Th,and 236U are 4.002604,232.038124,and 236.045637 amu,respectively.
A)1)473 × 108 kJ/mol
B)4)418 × 108 kJ/mol
C)4)909 × 108 kJ/mol
D)7)209 × 108 kJ/mol
A)1)473 × 108 kJ/mol
B)4)418 × 108 kJ/mol
C)4)909 × 108 kJ/mol
D)7)209 × 108 kJ/mol

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
8
The sum of the masses of the protons and neutrons in a given nucleus minus the mass of that nucleus is always
A)negative.
B)positive.
C)temperature dependent.
D)zero.
A)negative.
B)positive.
C)temperature dependent.
D)zero.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
9
Breaking a chemical bond requires approximately 102 kJ/mol of energy.What is closest to the order of magnitude of the energy required to split a nucleus in to its individual protons and neutrons?
A)103 kJ/mol
B)104 kJ/mol
C)108 kJ/mol
D)1010 kJ/mol
A)103 kJ/mol
B)104 kJ/mol
C)108 kJ/mol
D)1010 kJ/mol

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
10
Calculate the mass defect for the formation of phosphorus-31.The mass of a phosphorus-31 nucleus is 30.973765 amu.The masses of a proton and a neutron are 1.00728 and 1.00866 amu,respectively.
A)0)13015 amu
B)0)15404 amu
C)0)27261 amu
D)0)27399 amu
A)0)13015 amu
B)0)15404 amu
C)0)27261 amu
D)0)27399 amu

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
11
If the mass of one neutron is 1.00866 amu,the mass of one proton is 1.00728 amu,and the mass of 12C nucleus is 11.99671 amu,calculate the binding energy for the 12C nucleus.
A)8)90 × 109 kJ/mol
B)8)90 × 1012 kJ/mol
C)1)10 × 1015 kJ/mol
D)1)10 × 1018 kJ/mol
A)8)90 × 109 kJ/mol
B)8)90 × 1012 kJ/mol
C)1)10 × 1015 kJ/mol
D)1)10 × 1018 kJ/mol

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
12
The binding energy is defined as
A)the amount of energy absorbed when electrons are added to an ion.
B)the amount of energy absorbed when protons and neutrons form a nucleus.
C)the amount of energy released when electrons are removed from the atom.
D)the amount of energy required to break apart a nucleus into individual protons and neutrons.
A)the amount of energy absorbed when electrons are added to an ion.
B)the amount of energy absorbed when protons and neutrons form a nucleus.
C)the amount of energy released when electrons are removed from the atom.
D)the amount of energy required to break apart a nucleus into individual protons and neutrons.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
13
Elements with ________ atomic mass are best possible candidates for a fusion reaction.
A)very low
B)moderate
C)moderate to heavy
D)very heavy
A)very low
B)moderate
C)moderate to heavy
D)very heavy

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
14
Which does not apply to a nuclear fusion reaction?
A)Atoms of one element cannot be converted to atoms of another element.
B)Energy is conserved.
C)Matter is conserved.
D)None of these apply to a nuclear fusion reaction.
A)Atoms of one element cannot be converted to atoms of another element.
B)Energy is conserved.
C)Matter is conserved.
D)None of these apply to a nuclear fusion reaction.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
15
Elements with ________ atomic mass are best possible candidates for a fission reaction.
A)very low
B)moderate
C)moderate to heavy
D)very heavy
A)very low
B)moderate
C)moderate to heavy
D)very heavy

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
16
A binding energy curve is a plot of binding energy per nucleon versus atomic number.In what region of the binding energy curve are the most stable elements found?
A)in the lower left region (low atomic mass)
B)in the central top region (moderate atomic mass)
C)in the lower right region (heavy atomic mass)
D)binding energy is not dependent on atomic mass
A)in the lower left region (low atomic mass)
B)in the central top region (moderate atomic mass)
C)in the lower right region (heavy atomic mass)
D)binding energy is not dependent on atomic mass

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
17
Four heavy elements (A,B,C,and D)will fission when bombarded by neutrons.In addition to fissioning into two smaller elements,A also gives off a beta particle,B gives off gamma rays,C gives off neutrons,and D gives off alpha particles.Which element would make a possible fuel for a nuclear reactor?
A)element A
B)element B
C)element C
D)element D
A)element A
B)element B
C)element C
D)element D

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
18
Which reaction is expected to release the greatest amount of energy?
A)2 H → H2
B)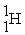 +
+ 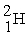 →
→ 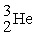
C)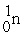 +
+ 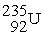 →
→ 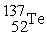 +
+ 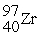 + 2 1n
+ 2 1n
D)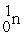 +
+ 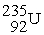 →
→ 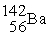 +
+ 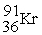 + 3
+ 3 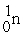
A)2 H → H2
B)
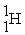 +
+ 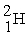 →
→ 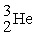
C)
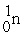 +
+ 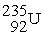 →
→ 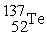 +
+ 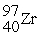 + 2 1n
+ 2 1nD)
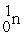 +
+ 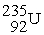 →
→ 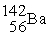 +
+ 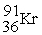 + 3
+ 3 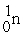

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
19
Relative to the amount of energy released in a typical chemical reaction,the amount of energy released in a typical fission reaction is about
A)the same.
B)10 times greater.
C)108 times greater
D)1010 times greater
A)the same.
B)10 times greater.
C)108 times greater
D)1010 times greater

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
20
What is the mass change in grams for the reaction 2 NO2(g) N2O4(g)? The enthalpy for the reaction is -57.2 kJ.
A)-6.36 × 10-13 g
B)-6.36 × 10-10 g
C)-1.91 × 10-7 g
D)-1.91 × 10-4 g
A)-6.36 × 10-13 g
B)-6.36 × 10-10 g
C)-1.91 × 10-7 g
D)-1.91 × 10-4 g

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
21
The masses of 4He,6Li,and 10B are 4.0015,6.0135,and 10.0102 amu respectively.The fission of a boron-10 nucleus into He-4 and Li-6 would
A)absorb energy.
B)evolve energy.
C)result in no energy change.
D)Need more information
A)absorb energy.
B)evolve energy.
C)result in no energy change.
D)Need more information

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
22
The nuclear transmutation reaction that leads to the synthesis of plutonium-241 is shown below.What is the identity of the α-particle bombardment target in this reaction?
? +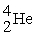
→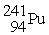
+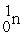
A)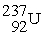
B)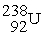
C)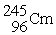
D)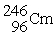
? +
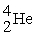
→
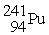
+
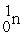
A)
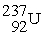
B)
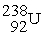
C)
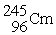
D)
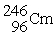

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
23
The first transuranium element was synthesized by bombarding 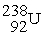
With neutrons.After capturing one neutron,the resulting nuclide was unstable and decayed by beta emission.What was the product of these two nuclear reactions?
A)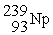
B)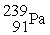
C)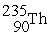
D)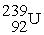
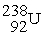
With neutrons.After capturing one neutron,the resulting nuclide was unstable and decayed by beta emission.What was the product of these two nuclear reactions?
A)
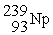
B)
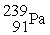
C)
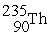
D)
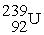

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
24
Nitrogen-14 (14.003074 amu)is synthesized in the sun by fusion of 13C (13.003355 amu)and 1H (1.007825 amu).How much energy is released in this nuclear reaction?
A)2)432 × 106 kJ/mol
B)7)295 × 108 kJ/mol
C)9)141 × 1010 kJ/mol
D)1)807 × 1011 kJ/mol
A)2)432 × 106 kJ/mol
B)7)295 × 108 kJ/mol
C)9)141 × 1010 kJ/mol
D)1)807 × 1011 kJ/mol

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
25
What does the term "critical mass" mean? It is the
A)amount of fissionable material which will self-sustain a nuclear chain reaction.
B)difference between mass of individual protons and neutrons and the mass of the nucleus.
C)energy which holds the nucleus together.
D)mass of fuel in a nuclear reactor core.
A)amount of fissionable material which will self-sustain a nuclear chain reaction.
B)difference between mass of individual protons and neutrons and the mass of the nucleus.
C)energy which holds the nucleus together.
D)mass of fuel in a nuclear reactor core.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
26
Which one of the following instruments would be the least suitable for detecting particles given off in radioactive decay?
A)electron microsope
B)Geiger counter
C)photographic film
D)scintillation counter
A)electron microsope
B)Geiger counter
C)photographic film
D)scintillation counter

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
27
Which ionizing radiation has the greatest energy?
A)α
B)β
C)γ
D)χ
A)α
B)β
C)γ
D)χ

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
28
A shorthand way of writing transmutation equations is to show the bombarding particle and the subsequent emitted particle in parentheses.Which particle is produced by
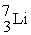
(p,α)?
A)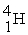
B)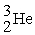
C)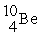
D)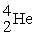
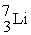
(p,α)?
A)
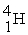
B)
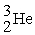
C)
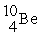
D)
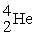

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
29
What mass percent of 235U (the fissionable isotope)is used in the fuel in nuclear power plants?
A)3%
B)14%
C)27%
D)83%
A)3%
B)14%
C)27%
D)83%

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
30
Oxygen-16 (15.994915 amu)is synthesized in the sun by fusion of 12C (12.000000 amu)and 4He (4.00260 amu).How much energy is released in this nuclear reaction?
A)2)48 × 103 kJ/mol
B)2)30 × 106 kJ/mol
C)6)92 × 108 kJ/mol
D)7)20 × 1011 kJ/mol
A)2)48 × 103 kJ/mol
B)2)30 × 106 kJ/mol
C)6)92 × 108 kJ/mol
D)7)20 × 1011 kJ/mol

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
31
In fusion reactions to make superheavy elements,energy is required to bring the two nuclei together and to form additional mass.How much energy must be converted into mass for the alpha bombardment of 253Es to form 257Md? (The isotopic masses are 253Es = 253.08294 amu,4He = 4.00260 amu,257Md = 257.09558 amu. )
A)3)01 × 106 kJ/mol
B)9)04 × 108 kJ/mol
C)3)59 × 1011 kJ/mol
D)7)21 × 1011 kJ/mol
A)3)01 × 106 kJ/mol
B)9)04 × 108 kJ/mol
C)3)59 × 1011 kJ/mol
D)7)21 × 1011 kJ/mol

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
32
Radiation is detected by its ________ properties.
A)gravitational
B)ionizing
C)kinetic
D)thermal
A)gravitational
B)ionizing
C)kinetic
D)thermal

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
33
When 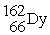
Is formed by bombardment of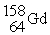
,what particle(s)was (were)used for the bombardment?
A)one α
B)two β-
C)4 neutrons
D)2 protons
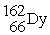
Is formed by bombardment of
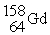
,what particle(s)was (were)used for the bombardment?
A)one α
B)two β-
C)4 neutrons
D)2 protons

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
34
A curie is:
A)the amount of sample that undergoes 1 disintegration per second.
B)the amount of sample that undergoes 3.7 × 1010 disintegrations per second.
C)the amount of tissue damage done by radiation.
D)equal to 0.01 J of energy absorbed per kilogram of tissue.
A)the amount of sample that undergoes 1 disintegration per second.
B)the amount of sample that undergoes 3.7 × 1010 disintegrations per second.
C)the amount of tissue damage done by radiation.
D)equal to 0.01 J of energy absorbed per kilogram of tissue.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
35
A sievert is:
A)the amount of sample that undergoes 1 disintegration per second.
B)the amount of sample that undergoes 3.7 × 1010 disintegrations per second.
C)the amount of tissue damage done by radiation.
D)equal to 0.01 J of energy absorbed per kilogram of tissue.
A)the amount of sample that undergoes 1 disintegration per second.
B)the amount of sample that undergoes 3.7 × 1010 disintegrations per second.
C)the amount of tissue damage done by radiation.
D)equal to 0.01 J of energy absorbed per kilogram of tissue.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
36
A becquerel is:
A)the amount of sample that undergoes 1 disintegration per second.
B)the amount of sample that undergoes 3.7 × 1010 disintegrations per second.
C)the amount of tissue damage done by radiation.
D)equal to 0.01 J of energy absorbed per kilogram of tissue.
A)the amount of sample that undergoes 1 disintegration per second.
B)the amount of sample that undergoes 3.7 × 1010 disintegrations per second.
C)the amount of tissue damage done by radiation.
D)equal to 0.01 J of energy absorbed per kilogram of tissue.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
37
A shorthand way of writing transmutation equations is to show the bombarding particle and the subsequent emitted particle in parentheses.Which of the following equations shows the balanced nuclear reaction for 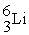
(n,α)?
A)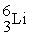 +
+ 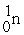 →
→ 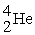 +
+ 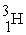
B)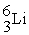 +
+ 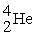 →
→ 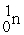 +
+ 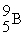
C)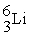 +
+ 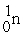 →
→ 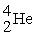 +
+ 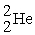
D)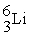 +
+ 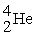 →
→ 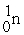 +
+ 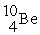
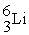
(n,α)?
A)
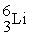 +
+ 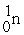 →
→ 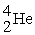 +
+ 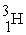
B)
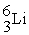 +
+ 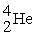 →
→ 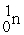 +
+ 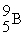
C)
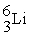 +
+ 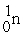 →
→ 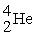 +
+ 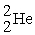
D)
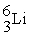 +
+ 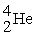 →
→ 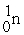 +
+ 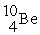

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
38
Which one of the following is most penetrating?
A)alpha particles
B)beta particles
C)gamma rays
D)positrons
A)alpha particles
B)beta particles
C)gamma rays
D)positrons

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following reactions correctly shows the transmutation of an element by neutron absorption followed by beta emission?
A)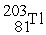 +
+ 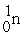 →
→ 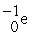 +
+ 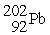
B)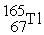 +
+ 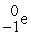 →
→ 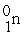 +
+ 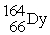
C)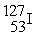 +
+ 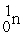 →
→ 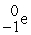 +
+ 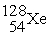
D)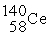 +
+ 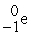 →
→ 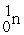 +
+ 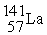
A)
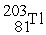 +
+ 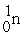 →
→ 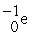 +
+ 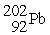
B)
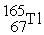 +
+ 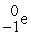 →
→ 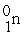 +
+ 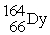
C)
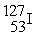 +
+ 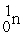 →
→ 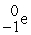 +
+ 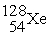
D)
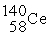 +
+ 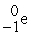 →
→ 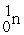 +
+ 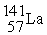

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
40
What is the missing product of the nuclear reaction? 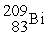
+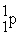
→ ? +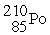
A)α
B)β+
C)β-
D)n
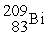
+
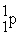
→ ? +
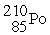
A)α
B)β+
C)β-
D)n

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
41
A few sheets of ordinary paper can form an effective shield against what type of radiation?
A)alpha particles
B)cosmic rays
C)gamma rays
D)neutrons
A)alpha particles
B)cosmic rays
C)gamma rays
D)neutrons

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
42
A small block of wood or heavy clothing can form an effective shield against what type of radiation?
A)beta particles
B)cosmic rays
C)gamma rays
D)neutrons
A)beta particles
B)cosmic rays
C)gamma rays
D)neutrons

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
43
An archeological artifact was subjected to radiocarbon dating.The artifact showed a carbon-14 decay rate of 13.8 disintegrations/min per gram of carbon.Carbon-14 has a half-life of 5715 years,and currently living organisms decay at the rate of 15.3 disintegrations/min per gram of carbon.What is the approximate age of the artifact?
A)257 years old
B)371 years old
C)591 years old
D)851 years old
A)257 years old
B)371 years old
C)591 years old
D)851 years old

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
44
Ionizing radiation having the lowest energy is exhibited by
A)α particles.
B)β particles.
C)γ rays.
D)X-rays
A)α particles.
B)β particles.
C)γ rays.
D)X-rays

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
45
Which is more dangerous in terms of short-lived exposure to radiation? One mole of
A)(89Sr,β-,t1/2 = 50.5 days.)
B)(90Sr,β-,t1/2 = 28.5 years.)
C)(91Sr,β-,t1/2 = 9.5 hours.)
D)(94Sr,β-,t1/2 = 74 seconds.)
A)(89Sr,β-,t1/2 = 50.5 days.)
B)(90Sr,β-,t1/2 = 28.5 years.)
C)(91Sr,β-,t1/2 = 9.5 hours.)
D)(94Sr,β-,t1/2 = 74 seconds.)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
46
Which unit of radiation is frequently used in medicine to describe the amount of tissue damage caused by a radioactive substance?
A)becquerel
B)curie
C)rad
D)rem
A)becquerel
B)curie
C)rad
D)rem

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
47
Which unit of radiation describes the amount of radiation emitted by a radioactive substance?
A)becquerel
B)gray
C)rad
D)rem
A)becquerel
B)gray
C)rad
D)rem

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
48
Carbon-14,which is present in all living tissue,radioactively decays via a first-order process.A one-gram sample of wood taken from a living tree gives a rate for carbon-14 decay of 13.6 counts per minute.If the half-life for carbon-14 is 5715 years,how old is a wood sample that gives a rate for carbon-14 decay of 3.9 counts per minute?
A)4)6 × 103 yr
B)6)6 × 103 yr
C)1)0 × 104 yr
D)2)9 × 104 yr
A)4)6 × 103 yr
B)6)6 × 103 yr
C)1)0 × 104 yr
D)2)9 × 104 yr

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
49
Which type of radiation is not used for medical applications?
A)alpha emission
B)beta emission
C)gamma radiation
D)positron emission
A)alpha emission
B)beta emission
C)gamma radiation
D)positron emission

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
50
The ratio of 238U to 206Pb is used to date old mineral samples.Each 1.000 g of 238U that decays eventually produces 0.866 g of 206Pb.If the half-life of 238U is 4.468 × 109 years,what is the age of a mineral that has a 238U/206Pb mass ratio of 2.00?
A)2)94 × 109 years
B)3)60 × 109 years
C)3)87 × 109 years
D)4)47 × 109 years
A)2)94 × 109 years
B)3)60 × 109 years
C)3)87 × 109 years
D)4)47 × 109 years

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
51
What type of shielding can be used to protect against gamma rays?
A)cloth
B)lead
C)wood
D)No shielding is effective against gamma rays.
A)cloth
B)lead
C)wood
D)No shielding is effective against gamma rays.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
52
What is the abbreviation used for the curie,a unit used in the measurement of nuclear disintegrations per unit time?
A)Cu
B)Cr
C)Ci
D)Ce
A)Cu
B)Cr
C)Ci
D)Ce

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
53
Most of the radiation to which people are exposed comes from
A)atmospheric testing of nuclear weapons.
B)emissions from nuclear power plants.
C)medical procedures.
D)natural sources.
A)atmospheric testing of nuclear weapons.
B)emissions from nuclear power plants.
C)medical procedures.
D)natural sources.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
54
If a log contains 60.0% of the 14C present in a living tree,how long has the log been dead? The half-life of 14C is 5730 years.
A)2290 years
B)3430 years
C)4220 years
D)7560 years
A)2290 years
B)3430 years
C)4220 years
D)7560 years

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
55
Which unit of radiation describes the amount of energy absorbed by a kilogram of tissue exposed to a radiation source?
A)becquerel
B)curie
C)rad
D)rem
A)becquerel
B)curie
C)rad
D)rem

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
56
Which type of radiation source would pose the greatest health risk when used internally for a medical procedure?
A)an alpha emitter,t1/2 = 6 hours
B)a beta emitter,t1/2 = 6 hours
C)a beta emitter,t1/2 = 15 days
D)a gamma emitter,t1/2 = 6 hours
A)an alpha emitter,t1/2 = 6 hours
B)a beta emitter,t1/2 = 6 hours
C)a beta emitter,t1/2 = 15 days
D)a gamma emitter,t1/2 = 6 hours

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
57
A rad is:
A)the amount of sample that undergoes 1 disintegration per second.
B)the amount of sample that undergoes 3.7 × 1010 disintegrations per second.
C)the amount of tissue damage done by radiation.
D)equal to 0.01 J of energy absorbed per kilogram of tissue.
A)the amount of sample that undergoes 1 disintegration per second.
B)the amount of sample that undergoes 3.7 × 1010 disintegrations per second.
C)the amount of tissue damage done by radiation.
D)equal to 0.01 J of energy absorbed per kilogram of tissue.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
58
Radiation is dangerous to organisms because
A)all radionuclides are poisonous.
B)it causes electrolysis of water in the cells.
C)it causes nuclear reactions in the cells.
D)it ionizes molecules in the cells.
A)all radionuclides are poisonous.
B)it causes electrolysis of water in the cells.
C)it causes nuclear reactions in the cells.
D)it ionizes molecules in the cells.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
59
The effects of ionizing radiation depend on
A)length of exposure to radiation.
B)location of source (internal or external).
C)type and energy of radiation.
D)All of these
A)length of exposure to radiation.
B)location of source (internal or external).
C)type and energy of radiation.
D)All of these

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following isotopes is not used for medical purposes?
A)(3H)
B)(14C)
C)(32P)
D)(60Co)
A)(3H)
B)(14C)
C)(32P)
D)(60Co)

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
61
Energy is ________ (released,required)in the transmutation reaction shown below.The atomic masses are 40Ar (39.9624),1H (1.0078),40K (39.9640),1n (1.0087). 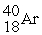
+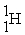
→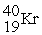
+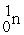
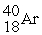
+
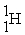
→
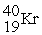
+
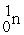

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
62
What is needed to balance the nuclear transmutation reaction below? 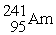 →
→ 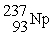
+ ?
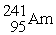 →
→ 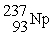
+ ?

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
63
If the age of the Earth is 4.5 billion years and the half-life of 40K is 1.26 billion years,what percent of the Earth's original amount of 40K remains today?
A)4)2%
B)8)4%
C)12%
D)16%
A)4)2%
B)8)4%
C)12%
D)16%

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
64
What is needed to balance the nuclear fusion reaction below?
2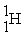 →
→ 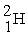 + ?
+ ?
2
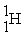 →
→ 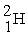 + ?
+ ?
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
65
The reaction below releases ________ kJ/mol.The atomic masses are 3He (3.0160),1H (1.0078),4He (4.0026),0e (0.0005486). 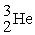
+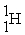
→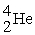
+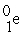
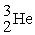
+
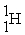
→
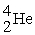
+
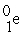

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
66
The binding energy for lithium-7 nuclei is 3.79 × 1012 J/mol.The binding energy per nucleon for lithium-7 nuclei is ________ MeV/nucleon.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
67
The mass defect for the chemical reaction shown below is ________ g.
2 F → F2 E = -159 kJ/mol
2 F → F2 E = -159 kJ/mol

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
68
What is needed to balance the nuclear fission reaction below? 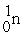 +
+ 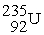 →
→ 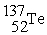
+ ? + 2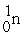
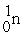 +
+ 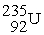 →
→ 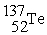
+ ? + 2
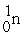

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
69
The mass change for the reaction shown below is ________ amu.The atomic masses are 3He (3.0160),4He (4.0026),1H (1.0078).
2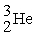
→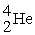
+ 2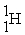
2
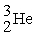
→
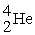
+ 2
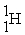

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
70
Carbon-14,which is present in all living tissue,radioactively decays via a first-order process.A one-gram sample of wood taken from a living tree gives a rate for carbon-14 decay of 13.6 counts per minute.If the half-life for carbon-14 is 5715 years,how old is a wood sample that gives a rate for carbon-14 decay of 11.9 counts per minute?
A)5)3 × 102 yr
B)7)6 × 102 yr
C)1)1 × 103 yr
D)9)4 × 103 yr
A)5)3 × 102 yr
B)7)6 × 102 yr
C)1)1 × 103 yr
D)9)4 × 103 yr

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
71
The mass of a helium-4 nucleus is ________ (greater than,less than,the same as)the sum of the masses of two protons plus two neutrons.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
72
The mass defect for the formation of lithium-6 is 0.0343 g/mol.The binding energy for lithium-6 nuclei is ________ kJ/mol.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
73
The practical limit for 14C dating occurs when its activity falls to 0.20% of its original value due to interference in detectors by natural background radiation.If the half-life is 5715 years,what is the maximum age of a sample that can be dated by 14C without interference?
A)1)3 × 104 years
B)2)9 × 104 years
C)5)1 × 104 years
D)2)9 × 106 years
A)1)3 × 104 years
B)2)9 × 104 years
C)5)1 × 104 years
D)2)9 × 106 years

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
74
In the transmutation reaction that results in the formation of berkelium-243,shown below,the nucleus that is bombarded by alpha particles is ________.
? +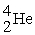 →
→ 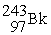 + 2
+ 2 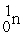
? +
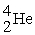 →
→ 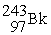 + 2
+ 2 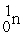

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
75
The loss in mass that occurs when protons and neutrons combine to form a nucleus is called the ________ of the nucleus,and the corresponding energy released during the formation of that nucleus is the ________ that holds the nucleus together.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
76
Radium occurs only in uranium ores,typically with an observed Ra/U ratio of 1mg/3kg.Uranium ores normally contain only about 200 ppm of U.How many kilograms of uranium ore must be processed to obtain 1 mg of radium?
A)1700 kg ore
B)15,000 kg ore
C)6)0 × 108 kg ore
D)None of these
A)1700 kg ore
B)15,000 kg ore
C)6)0 × 108 kg ore
D)None of these

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
77
The two reactions shown below are classified as (1)nuclear ________ and (2)nuclear ________.
(1)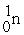
+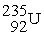
→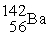
+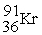
+ 3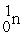
(2)
+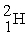
→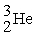
(1)
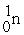
+
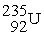
→
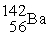
+
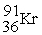
+ 3
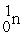
(2)

+
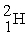
→
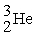

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
78
Among the types of radiation,α,β,γ,and X,the one that requires the least amount of protective clothing is ________ radiation.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
79
Helium-3,an electron,a neutron,and a proton have masses of 3.016029 amu,5.486 × 10-4 amu,1.00866 amu,and 1.00728 amu,respectively.The mass defect for the formation of helium-3 is ________ amu or ________ g/mol.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
80
Units used for measuring radiation include the becquerel,the curie,the gray,and the sievert,which have the abbreviations ________,________,________,and ________,respectively.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck



