Deck 2: The Chemistry of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/68
Play
Full screen (f)
Deck 2: The Chemistry of Life
1
The observations and research on aphid color changes can most directly be summarized in that
A) Koga and Fugatsu proved that the color change from red to green in aphids was ecologically favorable to survival.
B) species of organisms can be chemically diverse and affect each other, even among similar groups of aphids and bacteria.
C) the method of paper fiber separation of pigment molecules showed that Ricketsiella bacteria were the source of the green coloration of aphids.
D) it turned out that the green appearance of aphids was because of the large amount of green Ricketsiella bacteria coating their bodies.
A) Koga and Fugatsu proved that the color change from red to green in aphids was ecologically favorable to survival.
B) species of organisms can be chemically diverse and affect each other, even among similar groups of aphids and bacteria.
C) the method of paper fiber separation of pigment molecules showed that Ricketsiella bacteria were the source of the green coloration of aphids.
D) it turned out that the green appearance of aphids was because of the large amount of green Ricketsiella bacteria coating their bodies.
B
2
The initial experiment of Koga and Fugatsu,in testing for any bacterial cause of aphid color change,involved all of these except
A) the specific amounts of red and green pigment molecules were initially measured as dependent variables.
B) a group of aphids infected with Rickettsiella bacteria was grown, then killed in order to produce an extract to test on red aphids.
C) a group of green aphids was grown, then killed in order to produce an extract to test on red aphids.
D) a group of red aphids was grown as a control group.
E) a group of red aphids was treated with the independent variable of Rickettsiella bacteria infection from green aphids.
A) the specific amounts of red and green pigment molecules were initially measured as dependent variables.
B) a group of aphids infected with Rickettsiella bacteria was grown, then killed in order to produce an extract to test on red aphids.
C) a group of green aphids was grown, then killed in order to produce an extract to test on red aphids.
D) a group of red aphids was grown as a control group.
E) a group of red aphids was treated with the independent variable of Rickettsiella bacteria infection from green aphids.
A
3
The ring structure of glucose indicates that it is a(an)
A) disaccharide.
B) fatty acid.
C) monosaccharide.
D) nucleotide.
E) amino acid.
A) disaccharide.
B) fatty acid.
C) monosaccharide.
D) nucleotide.
E) amino acid.
C
4
In the 1700s,a French scientist,Antoine Lavoisier gained new experimental information about how chemistry works.He isolated chemicals that were reacting,including a metal and an acid.His observation of the results seemed to show that much of the metal had been lost in the chemical reaction.Yet,upon weighing the system,the total amounts of materials had not changed during the reaction.His resulting law of Conservation of Mass also applies to biology,because the materials we are made of are _________ that change forms,but aren't truly lost as we conduct life chemical reactions.
A) isotopes
B) energy
C) solutions
D) matter
A) isotopes
B) energy
C) solutions
D) matter

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
5
The diagram shows glucose and fructose before the chemical reaction called ______ builds a larger polymer from the two monomers.
A) evaporation
B) dehydration synthesis
C) hydrolysis
D) reproduction
A) evaporation
B) dehydration synthesis
C) hydrolysis
D) reproduction

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
6
The unique properties of water,including its strength as a solvent,its three environmental stages of solid,liquid,and gas,and its temperature regulation,are a result of
A) symmetric balance of electronegativity as shared electrons orbit equally around the hydrogens and oxygens.
B) the cohesion and adhesion of water molecules that bond more strongly to each other than other substances.
C) unbalanced electronegativity of the hydrogens and oxygens as they share electrons.
D) the imbalance in numbers of electrons around hydrogen and oxygen valence shells after they ionically bond.
A) symmetric balance of electronegativity as shared electrons orbit equally around the hydrogens and oxygens.
B) the cohesion and adhesion of water molecules that bond more strongly to each other than other substances.
C) unbalanced electronegativity of the hydrogens and oxygens as they share electrons.
D) the imbalance in numbers of electrons around hydrogen and oxygen valence shells after they ionically bond.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
7
Figuer:
Examine this image of the glucose molecule.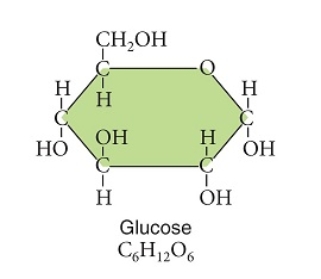
This glucose molecule is a(an)
A) triglyceride.
B) disaccharide.
C) polymer.
D) carbohydrate.
Examine this image of the glucose molecule.

This glucose molecule is a(an)
A) triglyceride.
B) disaccharide.
C) polymer.
D) carbohydrate.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
8
Figuer:
Examine these two sugars, as shown prior to the chemical reaction that would bond them.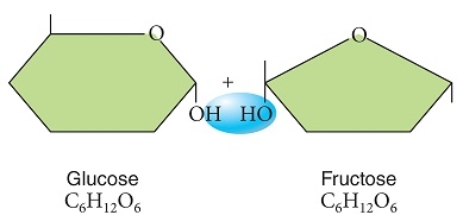
These glucose and fructose molecules will bond to form a monosaccharide with the removal of water.
Examine these two sugars, as shown prior to the chemical reaction that would bond them.

These glucose and fructose molecules will bond to form a monosaccharide with the removal of water.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
9
Researchers noted that only few aphids changed color to green from their original red.This is an unusual observation among any animals.What research question came out of the observations?
A) Will green aphids change their color to red, or remain green as they age?
B) Is the color shift of certain aphids due to genetics or some other factor?
C) The color shift of certain aphids is due to genetics within the species.
D) Do other aphids change colors as they age?
A) Will green aphids change their color to red, or remain green as they age?
B) Is the color shift of certain aphids due to genetics or some other factor?
C) The color shift of certain aphids is due to genetics within the species.
D) Do other aphids change colors as they age?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
10
Figuer:
Examine this image of the glucose molecule.
In our diets,this molecule is often covalently bonded with others in the polymer form of
A) a complex carbohydrate.
B) a fatty acid chain.
C) a triglyceride.
D) a simple sugar.
Examine this image of the glucose molecule.

In our diets,this molecule is often covalently bonded with others in the polymer form of
A) a complex carbohydrate.
B) a fatty acid chain.
C) a triglyceride.
D) a simple sugar.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
11
The atomic number of an element is the number of
A) protons in the orbitals.
B) neutrons in the orbitals.
C) neutrons in the nucleus.
D) protons in the nucleus.
E) electrons in the nucleus.
A) protons in the orbitals.
B) neutrons in the orbitals.
C) neutrons in the nucleus.
D) protons in the nucleus.
E) electrons in the nucleus.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
12
Compared with a molecule of glucose,this starch molecule does NOT have which characteristic below? 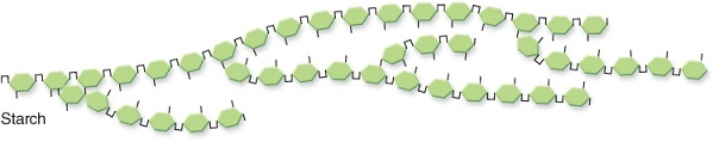
A) This molecule is used by cells for long-term storage and release of energy for cell functions.
B) This molecule is used by cells for quick release of energy for cell functions.
C) This molecule can provide structure for cells that contain it.
D) This molecule is a complex carbohydrate polymer.

A) This molecule is used by cells for long-term storage and release of energy for cell functions.
B) This molecule is used by cells for quick release of energy for cell functions.
C) This molecule can provide structure for cells that contain it.
D) This molecule is a complex carbohydrate polymer.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
13
Figuer:
Refer to this diagram with common examples of substances and their pH.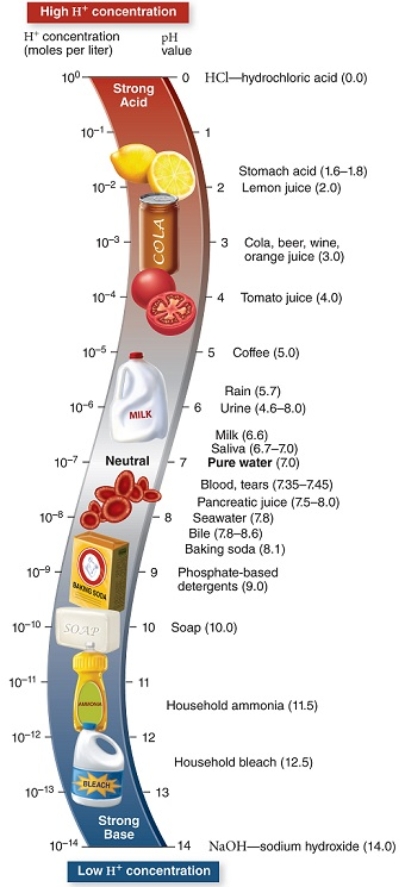
Our normal blood pH should be in a fairly narrow range.Imagine you sit down to eat a large meal with cola,tomato-based sauce,and a salad with many citrus fruit slices.Identify the one statement that does not apply as one of the likely outcomes of your meal.
A) The cola, tomato and citrus fruits will add hydrogen (H+) to your blood and body fluids.
B) Your blood and body fluids will likely become more basic, with higher pH than the normal range.
C) Your body will produce buffer molecules to help neutralize acids you ate, so your blood pH doesn't change much.
D) Your blood and body fluids will likely become more acidic, with lower pH than the normal range.
Refer to this diagram with common examples of substances and their pH.

Our normal blood pH should be in a fairly narrow range.Imagine you sit down to eat a large meal with cola,tomato-based sauce,and a salad with many citrus fruit slices.Identify the one statement that does not apply as one of the likely outcomes of your meal.
A) The cola, tomato and citrus fruits will add hydrogen (H+) to your blood and body fluids.
B) Your blood and body fluids will likely become more basic, with higher pH than the normal range.
C) Your body will produce buffer molecules to help neutralize acids you ate, so your blood pH doesn't change much.
D) Your blood and body fluids will likely become more acidic, with lower pH than the normal range.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
14
What is the link between colored pigment molecules and other organic molecules?
A) The DNA molecule genetic sequence regulates protein molecule function, which can specifically modify pigment structure that affects color.
B) In the case of the aphids, the pigment molecules of bacteria are genetically passed on to the DNA of infected aphids.
C) This one group of aphids can easily alter the pigment molecule structure by modifying its DNA nucleotide sequence and building new proteins.
D) Pigment molecules are complex, made up of all four of the other organic molecule groups.
A) The DNA molecule genetic sequence regulates protein molecule function, which can specifically modify pigment structure that affects color.
B) In the case of the aphids, the pigment molecules of bacteria are genetically passed on to the DNA of infected aphids.
C) This one group of aphids can easily alter the pigment molecule structure by modifying its DNA nucleotide sequence and building new proteins.
D) Pigment molecules are complex, made up of all four of the other organic molecule groups.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
15
A conscientious person habitually reads nutrition labels on food packages for weight watching and general health.The main nutritional molecules are made up of
A) buffers.
B) trace elements.
C) isotopes.
D) bulk elements.
A) buffers.
B) trace elements.
C) isotopes.
D) bulk elements.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
16
The primary elements making up living organisms are
A) carbon, hydrogen, oxygen, sulfur, nitrogen, and phosphorus.
B) carbon, hydrogen, iron, sulfur, sodium, and calcium.
C) carbon, oxygen, iron, chlorine, sulfur, and phosphorus.
D) carbon, hydrogen, oxygen, calcium, iron, and iodine.
E) carbon, oxygen, sulfur, calcium, iron, and phosphorus.
A) carbon, hydrogen, oxygen, sulfur, nitrogen, and phosphorus.
B) carbon, hydrogen, iron, sulfur, sodium, and calcium.
C) carbon, oxygen, iron, chlorine, sulfur, and phosphorus.
D) carbon, hydrogen, oxygen, calcium, iron, and iodine.
E) carbon, oxygen, sulfur, calcium, iron, and phosphorus.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
17
Figuer:
Refer to this diagram with common examples of substances and their pH.
The correct functions of your lungs contribute to the normal pH level of between 7.35 and 7.45.If your lungs do not exchange and remove carbon dioxide from your blood,the blood pH will change.A pH 6.4 reading of your blood indicates
A) a health problem due to the pH value being 10X higher H+ concentrations than normal in your body.
B) a health problem due to the pH value being 10X higher OH- concentrations than normal in your body.
C) a health problem due to the pH value being 2X higher OH- concentrations than normal in your body.
D) no health risk, as part of normal pH changes in your body that in this case bring it closer to neutral pH.
E) a health problem due to the pH value being 2X higher H+ concentrations than normal in your body.
Refer to this diagram with common examples of substances and their pH.

The correct functions of your lungs contribute to the normal pH level of between 7.35 and 7.45.If your lungs do not exchange and remove carbon dioxide from your blood,the blood pH will change.A pH 6.4 reading of your blood indicates
A) a health problem due to the pH value being 10X higher H+ concentrations than normal in your body.
B) a health problem due to the pH value being 10X higher OH- concentrations than normal in your body.
C) a health problem due to the pH value being 2X higher OH- concentrations than normal in your body.
D) no health risk, as part of normal pH changes in your body that in this case bring it closer to neutral pH.
E) a health problem due to the pH value being 2X higher H+ concentrations than normal in your body.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
18
An ion is an atom that has
A) a net negative or positive charge, with number of electrons different from number of protons.
B) a net positive charge.
C) the same number of electrons as it does protons.
D) a net negative charge.
E) a different number of neutrons from the number of protons.
A) a net negative or positive charge, with number of electrons different from number of protons.
B) a net positive charge.
C) the same number of electrons as it does protons.
D) a net negative charge.
E) a different number of neutrons from the number of protons.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
19
The mass number is defined as the total number of __________ of an atom.
A) neutrons and electrons
B) protons
C) protons, neutrons, and electrons
D) protons and neutrons
E) protons and electrons
A) neutrons and electrons
B) protons
C) protons, neutrons, and electrons
D) protons and neutrons
E) protons and electrons

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
20
Given this information from one element in the periodic table of elements,the number of neutrons and protons is 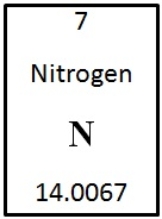
A) 7, which is the atomic number indicated.
B) 7, which is the atomic mass indicated.
C) 14, which is the atomic number indicated.
D) not discernable, because the number of electrons is also needed.
E) 14, which is the atomic mass indicated.

A) 7, which is the atomic number indicated.
B) 7, which is the atomic mass indicated.
C) 14, which is the atomic number indicated.
D) not discernable, because the number of electrons is also needed.
E) 14, which is the atomic mass indicated.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
21
The first energy shell of an atom contains a maximum of ________ electron(s).
A) one
B) two
C) eight
D) four
E) sixteen
A) one
B) two
C) eight
D) four
E) sixteen

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
22
In a chemical equation,which components of a chemical reaction is not noted in symbols and abbreviations?
A) Some substance atoms on the left side of the chemical reaction arrow must be destroyed in order to form the substances on the right side.
B) The reactants, or starting substances, are on the left of the chemical reaction arrow.
C) The products, or ending substances, are on the right of the chemical reaction arrow.
D) Reactants and products are on both sides of the yields arrow.
E) The number of atoms of each element must be the same, balanced on each side of the chemical reaction arrow.
A) Some substance atoms on the left side of the chemical reaction arrow must be destroyed in order to form the substances on the right side.
B) The reactants, or starting substances, are on the left of the chemical reaction arrow.
C) The products, or ending substances, are on the right of the chemical reaction arrow.
D) Reactants and products are on both sides of the yields arrow.
E) The number of atoms of each element must be the same, balanced on each side of the chemical reaction arrow.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
23
An acid
A) has a value above seven on the pH scale.
B) is a chemical that takes hydrogen ions from a solution.
C) has a value of seven on the pH scale.
D) is a chemical that adds hydrogen ions to a solution.
E) All of the answer choices are correct.
A) has a value above seven on the pH scale.
B) is a chemical that takes hydrogen ions from a solution.
C) has a value of seven on the pH scale.
D) is a chemical that adds hydrogen ions to a solution.
E) All of the answer choices are correct.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
24
You collect and measure samples of ice and surrounding ice water from a stream in the Winter.You find that you collected the same number of water molecules in each form.Water in the ice (solid)form floats in water of the liquid form because
A) in the ice form, the same number of water molecules are found in a crystal form, yet total larger volume than the liquid water.
B) in the ice form, the same number of water molecules are found in a solid, more compact volume than the liquid water.
C) once the water molecules froze into ice, they became hydrophobic, and started repelling from the liquid.
D) the ice is actively melting, since it was surrounded by the liquid water in the stream where it was collected.
A) in the ice form, the same number of water molecules are found in a crystal form, yet total larger volume than the liquid water.
B) in the ice form, the same number of water molecules are found in a solid, more compact volume than the liquid water.
C) once the water molecules froze into ice, they became hydrophobic, and started repelling from the liquid.
D) the ice is actively melting, since it was surrounded by the liquid water in the stream where it was collected.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
25
Within a single molecule of water,as shown,____ bonds are formed between oxygen and hydrogen. 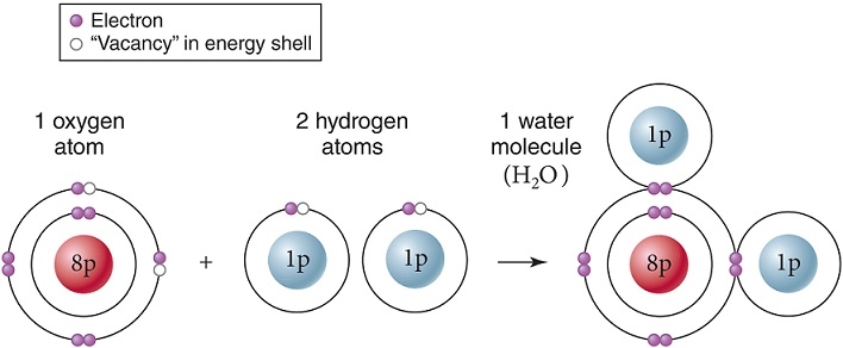
A) hydrophobic
B) hydrogen
C) nuclear
D) ionic
E) covalent

A) hydrophobic
B) hydrogen
C) nuclear
D) ionic
E) covalent

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
26
An element is found to have atoms with eight electrons in its valence shell.The atoms will be ____
A) highly reactive.
B) not chemically stable.
C) chemically stable.
D) highly likely to combine with other atoms.
E) not inert.
A) highly reactive.
B) not chemically stable.
C) chemically stable.
D) highly likely to combine with other atoms.
E) not inert.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
27
Trees are able to transport water from the roots to the top branches because
A) cohesion bonds water molecules to each other strongly.
B) adhesion bonds water molecules to the insides of the plant cells.
C) liquid water has a higher density than the air in the plant cells of the roots, trunk and branches.
D) water acts as a solvent of the tree cells as it moves upwards to the branches.
A) cohesion bonds water molecules to each other strongly.
B) adhesion bonds water molecules to the insides of the plant cells.
C) liquid water has a higher density than the air in the plant cells of the roots, trunk and branches.
D) water acts as a solvent of the tree cells as it moves upwards to the branches.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
28
The property of water demonstrated by this water strider,as it remains on top of the water,is that water is a universal solvent. 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
29
Carbon and hydrogen make up many biologically important molecules.Carbon has an electronegativity of 2.55 while hydrogen has an electronegativity of 2.0.On the scale of electronegativity from zero (0)to four (4),the carbon and hydrogens shown here have just formed 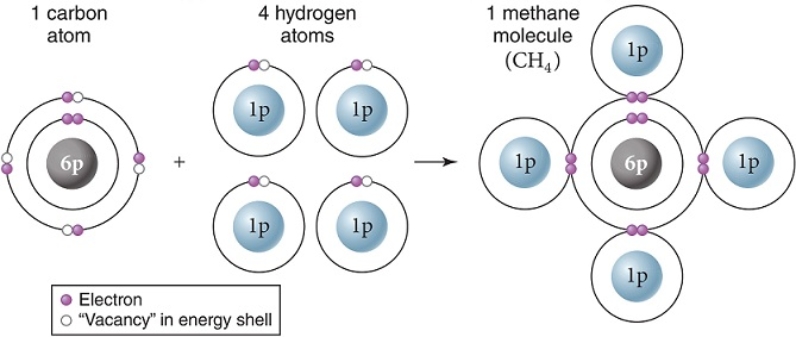
A) a nonpolar covalent bond.
B) a hydrogen bond.
C) a polar covalent bond.
D) an ionic bond.

A) a nonpolar covalent bond.
B) a hydrogen bond.
C) a polar covalent bond.
D) an ionic bond.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
30
Algal phytoplankton are single-celled water organisms that can do photosynthesis like plants.In a lake,summer growth of phytoplankton can change the water pH from pH 7.2 to 6.2.This change indicates all of these except
A) the water at pH 6.2 is a stronger acid solution than before the phytoplankton growth.
B) the water at pH 6.2 has ten times the hydrogen (H+) concentration as before the phytoplankton growth.
C) the water at pH 6.2 has twice the hydrogen (H+) concentration as before the phytoplankton growth.
D) the lake water solution changed from slightly basic to slightly acidic in pH.
A) the water at pH 6.2 is a stronger acid solution than before the phytoplankton growth.
B) the water at pH 6.2 has ten times the hydrogen (H+) concentration as before the phytoplankton growth.
C) the water at pH 6.2 has twice the hydrogen (H+) concentration as before the phytoplankton growth.
D) the lake water solution changed from slightly basic to slightly acidic in pH.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
31
In the example of ionic bond formation between sodium and chlorine,as shown,which of the following is not a true statement? 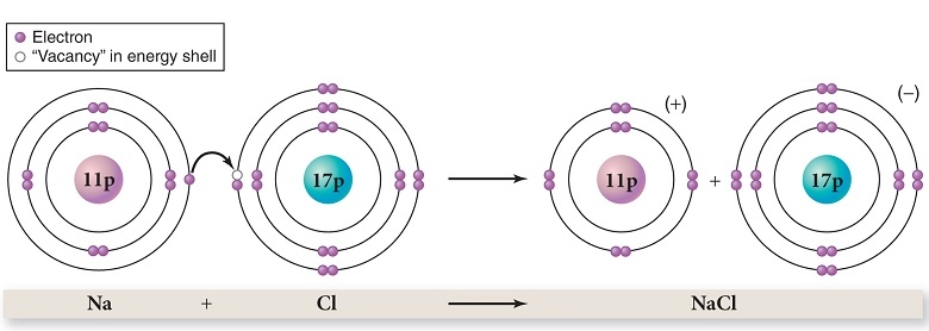
A) Sodium donates an electron.
B) The bond that is formed is stronger than a hydrogen bond.
C) Na is the chemical symbol for sodium.
D) Sodium becomes positively charged.
E) Chlorine donates an electron.

A) Sodium donates an electron.
B) The bond that is formed is stronger than a hydrogen bond.
C) Na is the chemical symbol for sodium.
D) Sodium becomes positively charged.
E) Chlorine donates an electron.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
32
In a covalent bond,atoms
A) share a proton.
B) share electrons.
C) of opposite charges attract each other.
D) both become highly electronegative.
A) share a proton.
B) share electrons.
C) of opposite charges attract each other.
D) both become highly electronegative.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
33
You can painlessly wade into a pool,but doing a belly flop off of the high diving board hurts because of
A) water's neutral pH.
B) adhesion in water.
C) cohesion in water.
D) water's high density.
E) water's high boiling point.
A) water's neutral pH.
B) adhesion in water.
C) cohesion in water.
D) water's high density.
E) water's high boiling point.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
34
A base
A) is a chemical that absorbs hydrogen ions from a solution.
B) has a value of 7 on the pH scale.
C) has a value below 7 on the pH scale.
D) is a chemical that adds hydrogen ions to a solution.
A) is a chemical that absorbs hydrogen ions from a solution.
B) has a value of 7 on the pH scale.
C) has a value below 7 on the pH scale.
D) is a chemical that adds hydrogen ions to a solution.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
35
Organic molecules are defined as chemical compounds that chiefly contain ______ in fairly distinct ratios and structures.
A) carbon and oxygen
B) carbon and nitrogen
C) carbon
D) carbon, hydrogen, and nitrogen
E) carbon and hydrogen
A) carbon and oxygen
B) carbon and nitrogen
C) carbon
D) carbon, hydrogen, and nitrogen
E) carbon and hydrogen

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
36
Evaporation of water is
A) a phase change of water from liquid into a vapor.
B) a phase change of water from solid into a vapor.
C) a phase change of water from vapor into a liquid.
D) a phase change of water from vapor into a solid.
E) All of the answer choices are correct.
A) a phase change of water from liquid into a vapor.
B) a phase change of water from solid into a vapor.
C) a phase change of water from vapor into a liquid.
D) a phase change of water from vapor into a solid.
E) All of the answer choices are correct.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
37
The chapter concept map links covalent and ionic bonds as chemical bonds that attract atoms or molecules.What is the chemical bond characteristic that contributes to the numerous important properties of water molecules for living organisms?
A) The covalent bond strengths of water molecules change with pH, temperature, or solute conditions present.
B) The covalent bonds that form water molecules transform to ionic bonds in presence of other molecules, temperature changes, or pH.
C) Hydrogen bonds form between water molecules, not requiring gain, loss, or sharing of electrons.
D) Bonds that form water are of the nonpolar covalent form.
A) The covalent bond strengths of water molecules change with pH, temperature, or solute conditions present.
B) The covalent bonds that form water molecules transform to ionic bonds in presence of other molecules, temperature changes, or pH.
C) Hydrogen bonds form between water molecules, not requiring gain, loss, or sharing of electrons.
D) Bonds that form water are of the nonpolar covalent form.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
38
Which statement summarizes the distinction between nonpolar and polar covalent bonds?
A) The electrons are more evenly and symmetrically distributed in orbit among atoms in a nonpolar covalent bond.
B) Polar covalent bonds are formed when the atoms gain or lose electrons to bond, and become oppositely charged ions.
C) The difference in electronegativity of the atoms in a nonpolar covalent bond is very large.
D) The electrons are more evenly and symmetrically distributed in orbit among atoms in a polar covalent bond.
A) The electrons are more evenly and symmetrically distributed in orbit among atoms in a nonpolar covalent bond.
B) Polar covalent bonds are formed when the atoms gain or lose electrons to bond, and become oppositely charged ions.
C) The difference in electronegativity of the atoms in a nonpolar covalent bond is very large.
D) The electrons are more evenly and symmetrically distributed in orbit among atoms in a polar covalent bond.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
39
If a molecule is added to a glass of water,and is easily dissolved by the water,the added molecule is described as hydrophilic.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
40
In an ionic bond,
A) two atoms both become strongly electronegative and attract each other.
B) atoms attract each other by sharing electrons to fill their valence shells.
C) atoms, having gained or lost electrons, attract one another with opposite charges.
D) two atoms are attracted by partial positive and negative charges.
A) two atoms both become strongly electronegative and attract each other.
B) atoms attract each other by sharing electrons to fill their valence shells.
C) atoms, having gained or lost electrons, attract one another with opposite charges.
D) two atoms are attracted by partial positive and negative charges.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
41
Saturated fats have long straight tails of fatty acids,and can pack or clump tightly together in cells and animal bodies.Unsaturated fats have kinks in their tails due to double bonds,which prevents them from packing together as tightly.Animals that are ectothermic (their body temperature fluctuates with the environment)need to keep their membranes fluid at cooler temperature and thus use ______ in their membranes.
A) mostly unsaturated fats
B) mostly saturated fats
C) equal amounts of saturated and unsaturated fats
D) carbohydrates
E) proteins
A) mostly unsaturated fats
B) mostly saturated fats
C) equal amounts of saturated and unsaturated fats
D) carbohydrates
E) proteins

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
42
Examples of monosaccharides are
A) glucose, maltose, and cellulose.
B) glucose, lactose, and maltose.
C) glucose, ribose, and fructose.
D) glucose, lactose, and cellulose.
E) None of these answers are correct; all options list lipids.
A) glucose, maltose, and cellulose.
B) glucose, lactose, and maltose.
C) glucose, ribose, and fructose.
D) glucose, lactose, and cellulose.
E) None of these answers are correct; all options list lipids.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
43
____ bonds are formed between monomers to form a polymer.
A) Hydrogen
B) Covalent
C) Nuclear
D) Hydrophobic
E) Ionic
A) Hydrogen
B) Covalent
C) Nuclear
D) Hydrophobic
E) Ionic

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
44
The comparison listed below that is not true in distinguishing DNA from RNA is that
A) DNA has a main function of storing our genetic code, while RNA is used in units to build specific proteins in a cell.
B) DNA is a long two-sided molecule while RNA is a shorter single-sided molecule.
C) DNA and RNA share all nucleotides, except that RNA has Uracil instead of Thymine.
D) DNA is a molecule that stores and regulates our genetics, while RNA is used for cellular energy storage and release for biological functions.
A) DNA has a main function of storing our genetic code, while RNA is used in units to build specific proteins in a cell.
B) DNA is a long two-sided molecule while RNA is a shorter single-sided molecule.
C) DNA and RNA share all nucleotides, except that RNA has Uracil instead of Thymine.
D) DNA is a molecule that stores and regulates our genetics, while RNA is used for cellular energy storage and release for biological functions.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
45
The primary building block (monomer)of nucleic acids is
A) a glucose molecule.
B) a fatty acid.
C) a nucleotide.
D) an amino acid.
E) a group of four interconnected rings.
A) a glucose molecule.
B) a fatty acid.
C) a nucleotide.
D) an amino acid.
E) a group of four interconnected rings.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
46
Many diseases,cancers and even normal human variations can be caused by mutations and variations in the DNA nucleotide sequence.The most likely immediate result of DNA having a different nucleotide sequence is that
A) the peptide bonds in the protein would by hydrolyzed and the protein would fall apart.
B) the protein resulting from the DNA mutation would be denatured and nonfunctional.
C) the primary structure of R group sequence in a protein would be altered.
D) no direct result of change in the protein molecule would occur if DNA is mutated.
A) the peptide bonds in the protein would by hydrolyzed and the protein would fall apart.
B) the protein resulting from the DNA mutation would be denatured and nonfunctional.
C) the primary structure of R group sequence in a protein would be altered.
D) no direct result of change in the protein molecule would occur if DNA is mutated.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
47
Cohesion is a property of water in which water molecules tend to stick together.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
48
A peptide bond is a covalent bond formed between the amino group of one amino acid and the R group of another amino acid.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
49
An amino acid contains a structural "backbone" chain of
A) nitrogens and carbons.
B) phosphorus atoms.
C) carbons.
D) nitrogens.
E) carbon and phosphorus atoms.
A) nitrogens and carbons.
B) phosphorus atoms.
C) carbons.
D) nitrogens.
E) carbon and phosphorus atoms.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
50
The primary building block (monomer)of proteins is
A) a nucleotide.
B) a fatty acid.
C) a glucose molecule.
D) a group of four interconnected rings.
E) an amino acid.
A) a nucleotide.
B) a fatty acid.
C) a glucose molecule.
D) a group of four interconnected rings.
E) an amino acid.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
51
The four nitrogen bases found in RNA are
A) adenine, thymine, guanine, and uracil.
B) adenine, cytosine, guanine, and uracil.
C) adenine, thymine, cytosine, and uracil.
D) thymine, cytosine, guanine, and uracil.
E) None of the answer choices are correct.
A) adenine, thymine, guanine, and uracil.
B) adenine, cytosine, guanine, and uracil.
C) adenine, thymine, cytosine, and uracil.
D) thymine, cytosine, guanine, and uracil.
E) None of the answer choices are correct.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
52
The group of organic molecule polymers with the most complex and diverse three-dimensional structure are
A) unsaturated fats.
B) proteins.
C) carbohydrates.
D) waxes.
E) saturated fats.
A) unsaturated fats.
B) proteins.
C) carbohydrates.
D) waxes.
E) saturated fats.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
53
The three major components in a nucleotide are
A) a nitrogen base, a five-carbon sugar, and a phosphate group.
B) a carboxyl group, an R group, and an amino group.
C) glucose, a nitrogen base, and a phosphate group.
D) a nitrogen base, a six-carbon sugar, and a phosphate group.
E) glucose, a fatty acid, and glycerol.
A) a nitrogen base, a five-carbon sugar, and a phosphate group.
B) a carboxyl group, an R group, and an amino group.
C) glucose, a nitrogen base, and a phosphate group.
D) a nitrogen base, a six-carbon sugar, and a phosphate group.
E) glucose, a fatty acid, and glycerol.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
54
Sugars (CH2O)n dissolve well in water because sugars form ____ bonds with water.
A) hydrophobic
B) hydrogen
C) non-polar
D) ionic
E) covalent
A) hydrophobic
B) hydrogen
C) non-polar
D) ionic
E) covalent

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
55
Saturated fats have long straight tails of fatty acids,while unsaturated fats from vegetables have kinks in their tails due to double bonds.These kinks prevent the fats from packing together as tightly.Hydrogenated vegetable oils,or trans fats,have hydrogens added back to the double bonds and thus behave like
A) carbohydrates.
B) waxes.
C) unsaturated fats.
D) saturated fats.
E) proteins.
A) carbohydrates.
B) waxes.
C) unsaturated fats.
D) saturated fats.
E) proteins.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
56
The four major groups of organic compounds are
A) carbohydrates, lipids, steroids, and monosaccharides.
B) lipids, fats, waxes, and steroids.
C) carbohydrates, proteins, amino acids, and nucleic acids.
D) carbohydrates, lipids, proteins, and nucleic acids.
E) fats, waxes, carbohydrates, and amino acids.
A) carbohydrates, lipids, steroids, and monosaccharides.
B) lipids, fats, waxes, and steroids.
C) carbohydrates, proteins, amino acids, and nucleic acids.
D) carbohydrates, lipids, proteins, and nucleic acids.
E) fats, waxes, carbohydrates, and amino acids.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
57
The bond that builds amino acid monomers into protein polymers is
A) a denatured hydrogen bond.
B) an ionic bond also known as a peptide bond.
C) a covalent bond also known as a peptide bond.
D) a primary structural bond.
A) a denatured hydrogen bond.
B) an ionic bond also known as a peptide bond.
C) a covalent bond also known as a peptide bond.
D) a primary structural bond.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
58
Blood pH is closely maintained at a pH of 7.4.A patient whose blood pH drops below 7.35 is suffering from metabolic acidosis and can go into a coma.What happens to the concentration of H+ ions in a patient with a blood pH of 6.4?
A) H+ concentration is increased 2-fold.
B) H+ concentration is decreased 2-fold.
C) H+ concentration is increased 10-fold.
D) H+ concentration is decreased 4-fold.
E) H+ concentration is decreased 10-fold.
A) H+ concentration is increased 2-fold.
B) H+ concentration is decreased 2-fold.
C) H+ concentration is increased 10-fold.
D) H+ concentration is decreased 4-fold.
E) H+ concentration is decreased 10-fold.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
59
In living cells,a process by which cells break polymers down into monomers by breaking covalent bonds is
A) hydrolysis.
B) dehydration synthesis.
C) reproduction.
D) All of the answer choices are correct.
A) hydrolysis.
B) dehydration synthesis.
C) reproduction.
D) All of the answer choices are correct.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
60
Which is not a lipid?
A) a triglyceride
B) a phospholipid
C) a wax
D) a sterol
E) a starch
A) a triglyceride
B) a phospholipid
C) a wax
D) a sterol
E) a starch

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
61
Among numerous functions of common proteins,which of these pairs does not correctly match a protein with its function?
A) DNA polymerase helps synthesize new DNA before our cells divide.
B) Antibodies regulate sweat to keep infections out of our skin pores.
C) Collagen is a structural protein to support hair, skin, and nails.
D) Insulin regulates blood glucose levels.
E) Hemoglobin protein transports oxygen to our cells.
A) DNA polymerase helps synthesize new DNA before our cells divide.
B) Antibodies regulate sweat to keep infections out of our skin pores.
C) Collagen is a structural protein to support hair, skin, and nails.
D) Insulin regulates blood glucose levels.
E) Hemoglobin protein transports oxygen to our cells.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
62
Having the typical ratio of carbon,hydrogen,and oxygen of carbohydrates,the chemical formula for glucose is
A) C12H22O11.
B) C6H6O12.
C) C6H12O6.
D) C12H6O12.
E) C6H6O6.
A) C12H22O11.
B) C6H6O12.
C) C6H12O6.
D) C12H6O12.
E) C6H6O6.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
63
Our general economic source of unsaturated fatty acids is from plants,and composed of at least one pair of double-bonded carbons.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
64
A substance in which other substances dissolve is called a solute.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
65
Of the 20 common amino acids in all organisms,essential amino acids are those we must consume in food.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
66
Proteins store the genetic information of the cell and transmit it to the next generation.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
67
If a carbohydrate polymer is limited to two monomer units,such as sucrose made from glucose and fructose,it is called
A) an oligosaccharide.
B) a disaccharide.
C) a polysaccharide.
D) a monosaccharide.
A) an oligosaccharide.
B) a disaccharide.
C) a polysaccharide.
D) a monosaccharide.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
68
If a protein is denatured,its structure has been changed enough to make the protein nonfunctional.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck



