Deck 4: Organic Compounds: Cycloalkanes and Their Stereochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/18
Play
Full screen (f)
Deck 4: Organic Compounds: Cycloalkanes and Their Stereochemistry
1
Which of the following statements is not true regarding the conformation of substituted cyclohexanes?
A)ring flip of substituted cyclohexanes flips the axial and equatorial positions of substituents
B)substituted cyclohexanes are destabilized by 1,3-diaxial interactions
C)the twist-boat conformation of cyclohexane is more stable than the chair conformation
D)the relative amount of two conformations of substituted cyclohexanes can be determined from the difference in strain energy
A)ring flip of substituted cyclohexanes flips the axial and equatorial positions of substituents
B)substituted cyclohexanes are destabilized by 1,3-diaxial interactions
C)the twist-boat conformation of cyclohexane is more stable than the chair conformation
D)the relative amount of two conformations of substituted cyclohexanes can be determined from the difference in strain energy
the twist-boat conformation of cyclohexane is more stable than the chair conformation
2
Which of the following structures represents trans-1,2-dimethylcyclohexane?
A)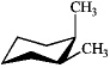
B)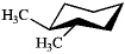
C)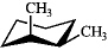
D)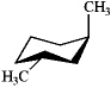
A)

B)

C)

D)


3
Substitution of which of the following groups on a cycloalkane would result in the greatest amount of steric strain?
A)bromo
B)ethyl
C)isopropyl
D)hydroxyl
A)bromo
B)ethyl
C)isopropyl
D)hydroxyl
isopropyl
4
Which one of the following structures represents a different compound from the other three?
A)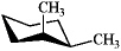
B)
C)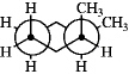
D)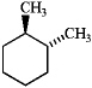
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following cycloalkanes would be the most similar to its open chain counterpart?
A)cyclodecane
B)cyclooctane
C)cyclopentane
D)cyclopropane
A)cyclodecane
B)cyclooctane
C)cyclopentane
D)cyclopropane

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is the most stable conformation of trans-1-ethyl-3-methylcyclohexane?
A)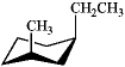
B)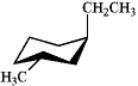
C)
D)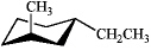
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
7
D-Pinitol is an interesting hexahydroxycyclohexane,whose structure is shown below.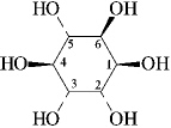
On the templates provided,draw the two chair conformations that are in equilibrium for D-pinitol.Circle the most stable conformation.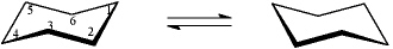

On the templates provided,draw the two chair conformations that are in equilibrium for D-pinitol.Circle the most stable conformation.


Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following structures represents trans-1,3-dimethylcyclohexane?
A)
B)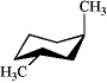
C)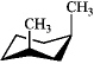
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
9
Which one of the following structures represents a different compound from the other three?
A)
B)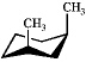
C)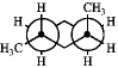
D)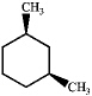
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
10
If the 1,3-diaxial strain for an ethyl group is 4.0 kJ/mol,what is the energy difference between the axial and equatorial conformations of ethylcyclohexane?
A)2.0 kJ/mol
B)4.0 kJ/mol
C)8.0 kJ/mol
D)16.0 kJ/mol
E)Cannot be determined from the 1,3-diaxial strain
A)2.0 kJ/mol
B)4.0 kJ/mol
C)8.0 kJ/mol
D)16.0 kJ/mol
E)Cannot be determined from the 1,3-diaxial strain

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the most stable conformation of cis-1-isopropyl-3-methylcyclohexane?
A)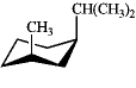
B)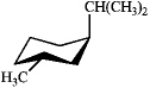
C)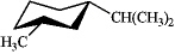
D)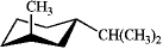
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following compounds can adopt a chair conformation in which there are no axial methyl groups?
A)1,1-dimethylcyclohexane
B)cis-1,2-dimethylcyclohexane
C)trans-1,2-dimethylcyclohexane
D)trans-1,3-dimethylcyclohexane
A)1,1-dimethylcyclohexane
B)cis-1,2-dimethylcyclohexane
C)trans-1,2-dimethylcyclohexane
D)trans-1,3-dimethylcyclohexane

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following cycloalkanes has the most ring strain?
A)cyclopropane
B)cyclobutane
C)cyclopentane
D)cyclohexane
A)cyclopropane
B)cyclobutane
C)cyclopentane
D)cyclohexane

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
14
Instructions: (-)-Menthol is responsible for the characteristic flavor and taste of peppermint.The structure of (-)-menthol is shown below.Use this information to answer the following question.
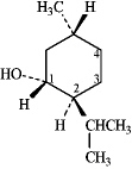
Refer to instructions.On the chair template provided below,draw the two chair conformations that are in equilibrium for (-)-menthol.

Refer to instructions.On the chair template provided below,draw the two chair conformations that are in equilibrium for (-)-menthol.


Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
15
In cycloheptane,which of the following factors contributes the least to the stability of the ring conformation?
A)torsional strain
B)angle strain
C)steric strain
D)all of these contribute to an approximately equal degree
A)torsional strain
B)angle strain
C)steric strain
D)all of these contribute to an approximately equal degree

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
16
In which of the following compounds would the carbon-carbon bond angle diverge the greatest from 109 ?
A)cyclodecane
B)cyclooctane
C)cyclopentane
D)cyclopropane
A)cyclodecane
B)cyclooctane
C)cyclopentane
D)cyclopropane

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
17
If cyclohexane were planar,how much torsional strain would be present in the planar molecule? Assume that the energy cost for each H«H eclipsing interaction is 4.0 kcal/mol.
A)12.0 kJ/mol
B)24.0 kJ/mol
C)36.0 kJ/mol
D)48.0 kJ/mol
E)64.0 kJ/mol
A)12.0 kJ/mol
B)24.0 kJ/mol
C)36.0 kJ/mol
D)48.0 kJ/mol
E)64.0 kJ/mol

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
18
What is the energy difference between the two chair conformations of the following compound that is due to steric strain?
Cis-1-bromo-4-isopropylcyclohexane

A)7.2 kJ/mol
B)5.6 kJ/mol
C)9.2 kJ/mol
D)11.2 kJ/mol
Cis-1-bromo-4-isopropylcyclohexane

A)7.2 kJ/mol
B)5.6 kJ/mol
C)9.2 kJ/mol
D)11.2 kJ/mol

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck



