Deck 8: Reactions of Alkenes and Alkynes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/37
Play
Full screen (f)
Deck 8: Reactions of Alkenes and Alkynes
1
Compare the products when the following compound reacts with Br2 in the presence of an organic solvent versus water.
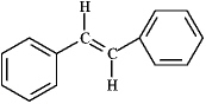

Organic solvent - bromination 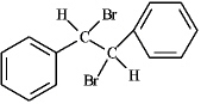
Water - halohydrin formation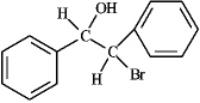 In an organic solvent halogenation of the alkene will occur producing a halogenated alkane,while in water (another nucleophile)the product formed would be the halohydrin.
In an organic solvent halogenation of the alkene will occur producing a halogenated alkane,while in water (another nucleophile)the product formed would be the halohydrin.

Water - halohydrin formation
 In an organic solvent halogenation of the alkene will occur producing a halogenated alkane,while in water (another nucleophile)the product formed would be the halohydrin.
In an organic solvent halogenation of the alkene will occur producing a halogenated alkane,while in water (another nucleophile)the product formed would be the halohydrin. 2
How many isomeric products would be formed when the following compound undergoes dehydration? 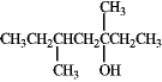
A)1
B)2
C)3
D)4
E)5

A)1
B)2
C)3
D)4
E)5
5
3
Instructions: Consider the reaction below to answer the following question(s).
Alkenes may be hydrated by the hydroboration/oxidation procedure shown.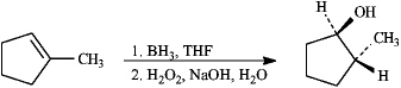
Refer to instructions.The regiochemistry of hydroboration/oxidation of alkenes is:
A)Markovnikov.
B)non-Markovnikov
C)subject to solvent effects.
D)unrelated to alkene structure.
Alkenes may be hydrated by the hydroboration/oxidation procedure shown.

Refer to instructions.The regiochemistry of hydroboration/oxidation of alkenes is:
A)Markovnikov.
B)non-Markovnikov
C)subject to solvent effects.
D)unrelated to alkene structure.
non-Markovnikov
4
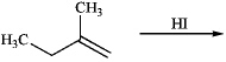
Predict the major product of the following reaction.


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
5
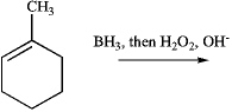
What is the major product of this reaction?


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
6
Predict the product of the reaction below.Indicate regiochemistry and stereochemistry if relevant.(D2 = deuterium)
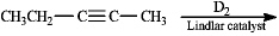
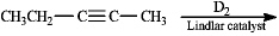

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
7
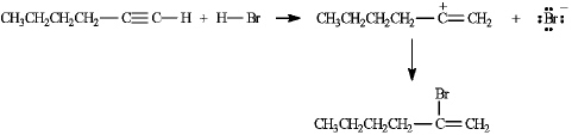
On the structures provided,draw arrows showing the electron flow in the reaction mechanism for the electrophilic addition of hydrogen bromide to hex-1-yne.


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
8
The reaction conditions to carry out the production of cyclopentene using bromocyclopentane as the starting material would be:
A)KOH,CH3CH2OH
B)H2SO4,THF
C)H2O2,OH-
D)Hg(OAc)2,H2O
A)KOH,CH3CH2OH
B)H2SO4,THF
C)H2O2,OH-
D)Hg(OAc)2,H2O

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
9
Instructions: The reaction of 2-methylpropene with HBr in ether gives one of the two products below.Answer the following question(s)about this reaction.
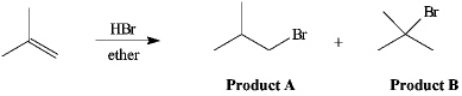
Refer to instructions.Which product would have a lower energy transition state for the formation of the intermediate leading to it?

Refer to instructions.Which product would have a lower energy transition state for the formation of the intermediate leading to it?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
10
The product(s)of the reaction when carried out in an organic solvent 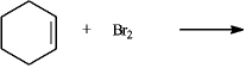
Would be:
A)cis-1,2-dibromocyclohexane only
B)trans-1,2-dibromocyclohexane only
C)50/50 mixture of cis-1,2-dibromocyclohexane and trans-1,2-dibromocyclohexane only
D)mixture with > 50% being trans-1,2-dibromocyclohexane only
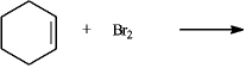
Would be:
A)cis-1,2-dibromocyclohexane only
B)trans-1,2-dibromocyclohexane only
C)50/50 mixture of cis-1,2-dibromocyclohexane and trans-1,2-dibromocyclohexane only
D)mixture with > 50% being trans-1,2-dibromocyclohexane only

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
11
Instructions: The reaction of 2-methylpropene with HBr in ether gives one of the two products below.Answer the following question(s)about this reaction.
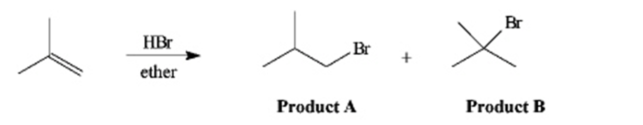
Refer to instructions. The reaction mixture would contain a majority of which isomeric product?

Refer to instructions. The reaction mixture would contain a majority of which isomeric product?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
12
Instructions: For each reaction below,suggest structures for alkenes that give the indicated reaction products.There may be more than one answer.
Suggest structure(s):
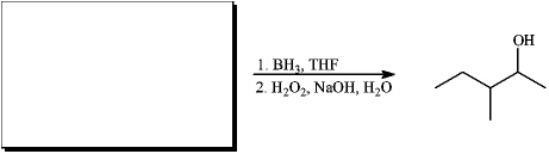
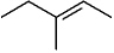
Suggest structure(s):



Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
13
Which set of reagents is used for the Markovnikov addition of water to an alkene without rearrangement?
A)BH3,THF followed by H2O2,NaOH
B)H2O,H2SO4
C)Hg(O2CCH3)2,H2O followed by NaBH4,NaOH
D)none of these
A)BH3,THF followed by H2O2,NaOH
B)H2O,H2SO4
C)Hg(O2CCH3)2,H2O followed by NaBH4,NaOH
D)none of these

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
14
Instructions: Consider the reaction sequence below to answer the following question.
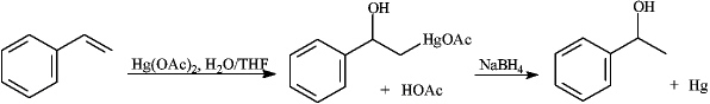
Refer to instructions.Write the complete reaction mechanism for the first step of this reaction sequence.Show all electron flow with arrows and show all intermediate structures.

Refer to instructions.Write the complete reaction mechanism for the first step of this reaction sequence.Show all electron flow with arrows and show all intermediate structures.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
15
Instructions: The reaction of 2-methylpropene with HBr in ether gives one of the two products below.Answer the following question(s)about this reaction.
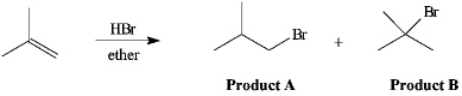
Refer to instructions.Which product is the Markovnikov product?

Refer to instructions.Which product is the Markovnikov product?

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
16
Instructions: Predict the products of each reaction below.Indicate regiochemistry and stereochemistry when relevant.
Predict and indicate:
a)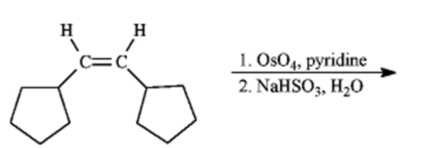
b) If the product of the above reaction were subsequently treated with periodic acid (HIO4), write the formula for the product formed.
Predict and indicate:
a)

b) If the product of the above reaction were subsequently treated with periodic acid (HIO4), write the formula for the product formed.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
17
Instructions: To answer the question(s)below,consider the following reaction:
When cyclohexene reacts with chlorine in tetrachloromethane,the trans-dihalide is formed.
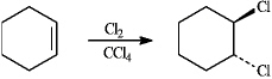
Refer to instructions.Write the complete,stepwise mechanism for this reaction.Show all intermediate structures and all electron flow using arrows.
When cyclohexene reacts with chlorine in tetrachloromethane,the trans-dihalide is formed.

Refer to instructions.Write the complete,stepwise mechanism for this reaction.Show all intermediate structures and all electron flow using arrows.

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
18
Instructions: Consider the reaction below to answer the following question(s).
Alkenes may be hydrated by the hydroboration/oxidation procedure shown.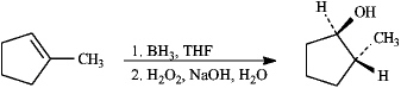
Refer to instructions.The intermediate formed in the first step of this reaction is:
A)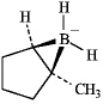
B)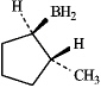
C)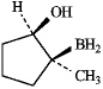
D)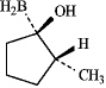
Alkenes may be hydrated by the hydroboration/oxidation procedure shown.

Refer to instructions.The intermediate formed in the first step of this reaction is:
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
19
Instructions: For each reaction below,suggest structures for alkenes that give the indicated reaction products.There may be more than one answer.
Suggest structure(s):
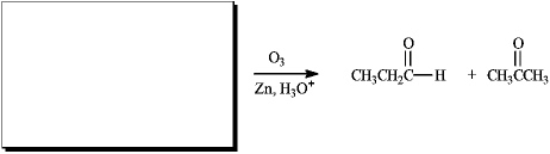
Suggest structure(s):


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
20
Instructions: Predict the products of each reaction below.Indicate regiochemistry and stereochemistry when relevant.
Predict and indicate:
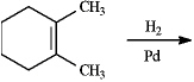
Predict and indicate:


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
21
What is the major organic product obtained from the following reaction?
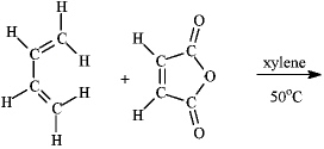


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
22
Draw all the isomeric products formed from the addition of HCl to the following compound.
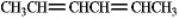
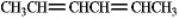

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
23
What type of reactive intermediate is involved in both of the following general reaction types? 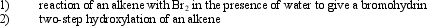
A)carbocation
B)carbanion
C)radical
D)cyclic bromonium ion
E)cyclic ion
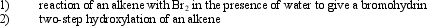
A)carbocation
B)carbanion
C)radical
D)cyclic bromonium ion
E)cyclic ion

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
24
What is the major organic product obtained from the following reaction?
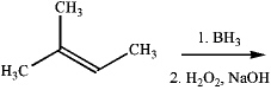


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
25
What type of reaction mechanism accounts for the reaction of an alkene with HBr to give an alkyl bromide?
A)nucleophilic addition
B)electrophilic addition
C)radical addition
D)elimination
A)nucleophilic addition
B)electrophilic addition
C)radical addition
D)elimination

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
26
Draw the structure of the polymer formed from the monomer given below,showing four repeating units.
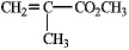
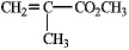

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
27
What type of reaction mechanism is involved in the reaction of an alcohol with aqueous acid to give an alkene?
A)nucleophilic addition
B)electrophilic addition
C)radical addition
D)elimination
A)nucleophilic addition
B)electrophilic addition
C)radical addition
D)elimination

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
28
Circle whichever of the following would be classified as a conjugated diene.
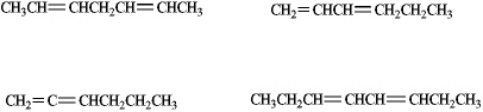
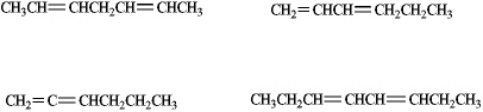

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
29
What type of reaction mechanism accounts for the reaction of an alkyne with HBr to give an alkyl bromide?
A)nucleophilic addition
B)electrophilic addition
C)radical addition
D)elimination
A)nucleophilic addition
B)electrophilic addition
C)radical addition
D)elimination

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
30
What is the major organic product obtained from the following reaction?
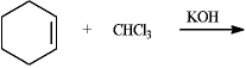
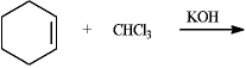

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
31
What type of reactive intermediate is formed in the reaction of an alkene with aqueous acid to give an alcohol?
A)carbocation
B)carbanion
C)radical
D)carbene
A)carbocation
B)carbanion
C)radical
D)carbene

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
32
What is the best choice of reagents to perform the following transformation? 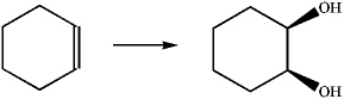
A)H2O,H2SO4
B)HgSO4;followed by NaBH4
C)BH3;followed by H2O2
D)OsO4;followed by NaHSO3

A)H2O,H2SO4
B)HgSO4;followed by NaBH4
C)BH3;followed by H2O2
D)OsO4;followed by NaHSO3

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
33
What is the major organic product obtained from the following reaction?
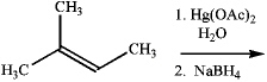


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
34
What type of reactive intermediate is formed in the reaction of an alkene with HBr to give a bromoalkane?
A)carbocation
B)carbanion
C)radical
D)cyclic bromonium ion
A)carbocation
B)carbanion
C)radical
D)cyclic bromonium ion

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
35
Complete the following reaction
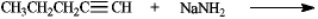
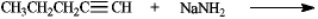

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
36
Draw the structure of the monomer used to prepare the polymer shown below.
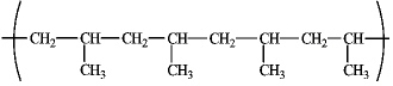


Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck
37
What is the best choice of reagents to perform the following transformation? 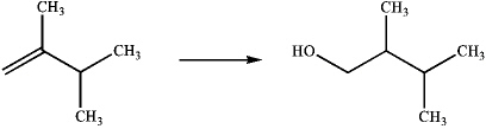
A)H2O,H2SO4
B)Hg(OAc)2 and H2O;followed by NaBH4
C)BH3;followed by H2O2,NaOH
D)OsO4;followed by NaHSO3

A)H2O,H2SO4
B)Hg(OAc)2 and H2O;followed by NaBH4
C)BH3;followed by H2O2,NaOH
D)OsO4;followed by NaHSO3

Unlock Deck
Unlock for access to all 37 flashcards in this deck.
Unlock Deck
k this deck



