Deck 6: An Overview of Organic Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/32
Play
Full screen (f)
Deck 6: An Overview of Organic Reactions
1
Which of the following correctly compares the two elements in terms of polarizability?
A)S > O
B)F > Br
C)N > P
D)Cl > I
A)S > O
B)F > Br
C)N > P
D)Cl > I
S > O
2
In the following generic reaction between a halogen (X)and an alkane (R),which of the following steps would be considered a chain termination step?
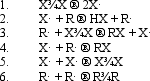
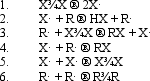
4,5,6
3
Instructions: The reaction below is commonly used as a laboratory preparation of cyclohexene.Use this reaction to answer the following question(s). 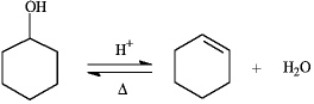
Refer to instructions.If this reaction under a given set of conditions has a Keq value of 5.67,what percent conversion to the product would be expected?
A)85%
B)5.67%
C)17%
D)15%
E)nearly 100%

Refer to instructions.If this reaction under a given set of conditions has a Keq value of 5.67,what percent conversion to the product would be expected?
A)85%
B)5.67%
C)17%
D)15%
E)nearly 100%
85%
4
Polarizability:
A)explains a nonpolar carbon-sulfur bond participating in polar reaction.
B)indicates the magnitude of a dipole moment.
C)is larger for smaller atoms with few electrons.
D)is the electric field associated with a polar bond.
E)all of these
A)explains a nonpolar carbon-sulfur bond participating in polar reaction.
B)indicates the magnitude of a dipole moment.
C)is larger for smaller atoms with few electrons.
D)is the electric field associated with a polar bond.
E)all of these

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
5
The structures below show the stepwise bond making and bond breaking in this reaction.Draw curved arrows to show the electron flow that has occurred in each step.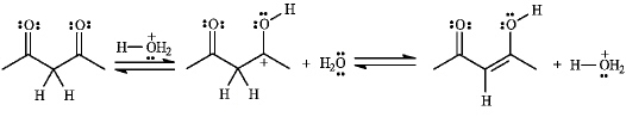


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
6
Instructions: Add curved arrows to the following reaction(s)to indicate the flow of electrons in each.
Indicate flow: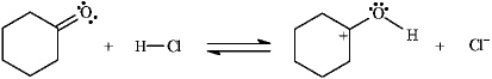
Indicate flow:
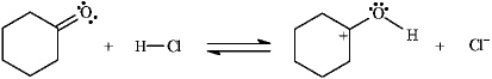

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
7
Instructions: Identify the functional groups present in each compound below,and predict the direction of polarity in each.
Identify and predict:
mustard gas Cl-CH2CH2-S-CH2CH2-Cl
Identify and predict:
mustard gas Cl-CH2CH2-S-CH2CH2-Cl

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
8
In the following generic reaction between a halogen (X)and an alkane (R),which of the following steps occurs slowly due to low concentration of reactants?
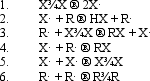
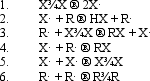

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
9
Instructions: Identify and label the nucleophile and electrophile in each reaction below.
Identify and label: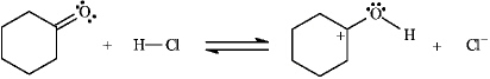
Identify and label:
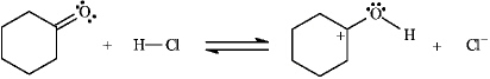

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
10
Instructions: The reaction below is commonly used as a laboratory preparation of cyclohexene.Use this reaction to answer the following question(s). 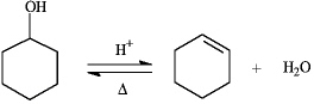
Refer to instructions.The forward and reverse reactions are classified,respectively,as:
A)addition,elimination
B)elimination,substitution
C)elimination,addition
D)elimination,rearrangement
E)substitution,addition

Refer to instructions.The forward and reverse reactions are classified,respectively,as:
A)addition,elimination
B)elimination,substitution
C)elimination,addition
D)elimination,rearrangement
E)substitution,addition

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
11
Instructions: Identify and label the nucleophile and electrophile in each reaction below.
Identify and label:
Identify and label:


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
12
In theory,upon reaction with water in the presence of a strong acid,which of the following will produce more than one isomeric product?
A)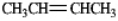
B)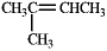
C)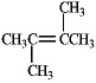
D)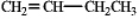
E)Both b and d
A)
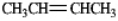
B)
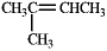
C)

D)
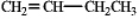
E)Both b and d

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
13
Instructions: Classify each structure below as a nucleophile or electrophile,and briefly explain your choice.
Classify and explain:
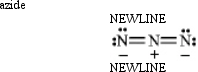
Classify and explain:
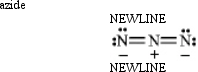

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
14
Instructions: Classify each structure below as a nucleophile or electrophile,and briefly explain your choice.
Classify and explain:
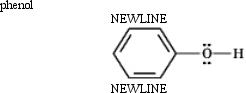
Classify and explain:


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
15
Instructions: Match each definition to one of the terms below.
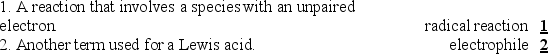
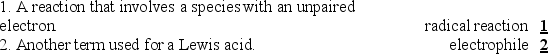

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
16
In an organic reaction,which of the following is most likely to function as only a nucleophile?
A)BF3
B)(CH3)2CH2NH2
C)Fe2+
D)CH3CH2S-
E)both a and c
A)BF3
B)(CH3)2CH2NH2
C)Fe2+
D)CH3CH2S-
E)both a and c

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
17
Instructions: Add curved arrows to the following reaction(s)to indicate the flow of electrons in each.
Indicate flow: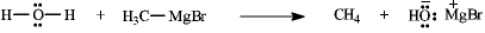
Indicate flow:
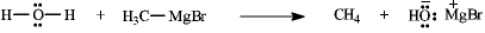

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
18
How many monochlorosubstitution products are possible for 2,3-dimethylbutane?
A)1
B)2
C)4
D)5
E)6
A)1
B)2
C)4
D)5
E)6

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is a characteristic of a polar reaction?
A)symmetrical bond making and breaking
B)one electron from each reactant forms the bond
C)involves a neutral species with an unpaired electron
D)are more common that radical reactions
E)all of these
A)symmetrical bond making and breaking
B)one electron from each reactant forms the bond
C)involves a neutral species with an unpaired electron
D)are more common that radical reactions
E)all of these

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
20
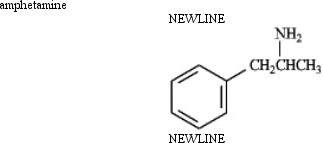
Instructions: Identify the functional groups present in each compound below,and predict the direction of polarity in each.
Identify and predict:
Identify and predict:


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
21
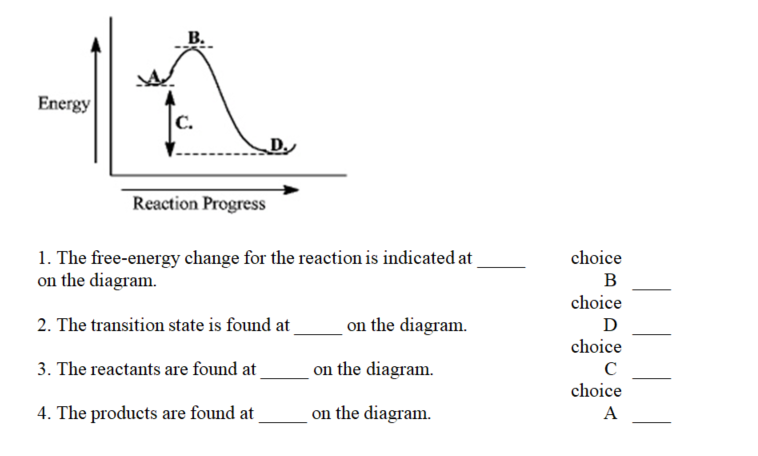
Instructions: Use the reaction energy diagram below to answer the following question(s).


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
22
Instructions: Use the second and third steps of the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).
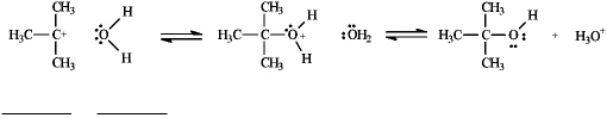
Refer to instructions.Label the nucleophile,Nu,and the electrophile,E+,in the blanks provided under the structures.

Refer to instructions.Label the nucleophile,Nu,and the electrophile,E+,in the blanks provided under the structures.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
23
Match each definition to one of the terms below.
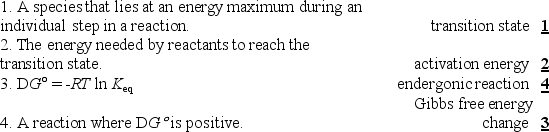
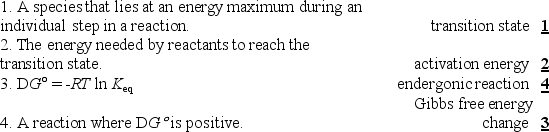

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
24
If the yield for the following reaction is 76%,calculate Keq and predict the sign of DG
.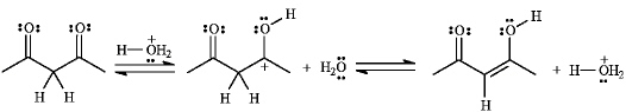
.


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
25
Instructions: Consider the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s). 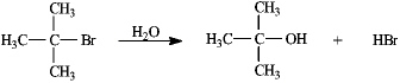
Refer to instructions.This reaction is an example of:
A)a substitution reaction.
B)a rearrangement reaction.
C)an elimination reaction.
D)an addition reaction.

Refer to instructions.This reaction is an example of:
A)a substitution reaction.
B)a rearrangement reaction.
C)an elimination reaction.
D)an addition reaction.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
26
Instructions: In the reaction below:
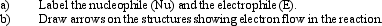
Label and indicate flow: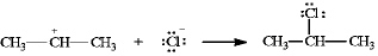
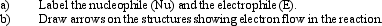
Label and indicate flow:
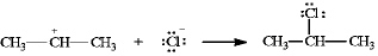

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
27
Consider the following reaction:
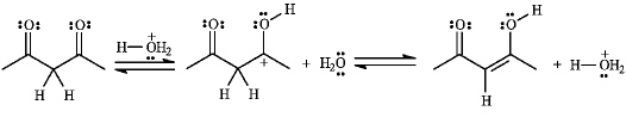
a)Assuming that the first step is the slow step, draw and label a qualitative energy diagram for the reaction.
b) If the second step of the reaction were the slow step, briefly explain how the values of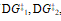 , and DG° would change.
, and DG° would change.

a)Assuming that the first step is the slow step, draw and label a qualitative energy diagram for the reaction.
b) If the second step of the reaction were the slow step, briefly explain how the values of
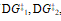 , and DG° would change.
, and DG° would change.
Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
28
Predict the product of the following reaction of Prostaglandin H2 by interpreting the flow of electrons as indicated by the curved arrows.


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
29
Instructions: Use the first step of the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s). 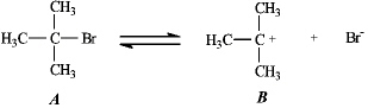
Refer to instructions.Species B is:
A)a carbocation
B)a radical
C)a carbanion
D)a free radical

Refer to instructions.Species B is:
A)a carbocation
B)a radical
C)a carbanion
D)a free radical

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
30
Predict the two alcohol addition products obtained by reaction of the following alkene with aqueous acid.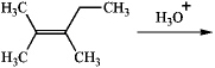


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
31
Instructions: Use the second and third steps of the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).
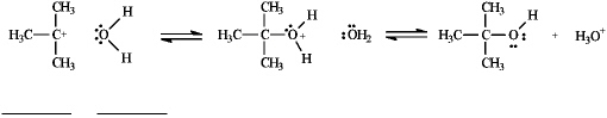
Refer to instructions.Draw arrows on the structures above showing electron flow in steps two and three of this reaction.

Refer to instructions.Draw arrows on the structures above showing electron flow in steps two and three of this reaction.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
32
Instructions: Use the first step of the reaction of 2-bromo-2-methylpropane with water,shown below,to answer the following question(s).
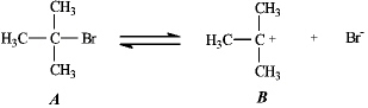
Refer to instructions.Add curved arrows to indicate electron flow in the first step.

Refer to instructions.Add curved arrows to indicate electron flow in the first step.

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck



