Deck 6: Thermochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/118
Play
Full screen (f)
Deck 6: Thermochemistry
1
Heat is
A)a measure of temperature.
B)a measure of the change in temperature.
C)a measure of thermal energy.
D)a measure of thermal energy transferred between two bodies at different temperature.
A)a measure of temperature.
B)a measure of the change in temperature.
C)a measure of thermal energy.
D)a measure of thermal energy transferred between two bodies at different temperature.
a measure of thermal energy transferred between two bodies at different temperature.
2
Suppose a 50.0 g block of silver (specific heat = 0.2350 J/g·°C)at 100°C is placed in contact with a 50.0 g block of iron (specific heat = 0.4494 J/g·°C)at 0°C, and the two blocks are insulated from the rest of the universe.The final temperature of the two blocks
A)will be higher than 50°C.
B)will be lower than 50°C.
C)will be exactly 50°C.
D)is unrelated to the composition of the blocks.
E)cannot be predicted.
A)will be higher than 50°C.
B)will be lower than 50°C.
C)will be exactly 50°C.
D)is unrelated to the composition of the blocks.
E)cannot be predicted.
will be lower than 50°C.
3
Three separate 3.5g blocks of Al, Cu, and Fe at 25 °C each absorb 0.505 kJ of heat.Which block reaches the highest temperature? The specific heats of Al, Cu, and Fe are 0.900 J/g·°C, 0.385J/g·°C, and 0.444 J/g·°C, respectively.
A)Al
B)Cu
C)Fe
D)Al and Cu
E)Fe and Cu
A)Al
B)Cu
C)Fe
D)Al and Cu
E)Fe and Cu
Cu
4
When 0.7521 g of benzoic acid was burned in a calorimeter containing 1,000.g of water, a temperature rise of 3.60°C was observed.What is the heat capacity of the bomb calorimeter, excluding the water? The heat of combustion of benzoic acid is -26.42 kJ/g.
A)1.34 kJ/°C
B)4.18 kJ/°C
C)5.52 kJ/°C
D)15.87 kJ/°C
E)752.1 kJ/°C
A)1.34 kJ/°C
B)4.18 kJ/°C
C)5.52 kJ/°C
D)15.87 kJ/°C
E)752.1 kJ/°C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
5
How much heat is required to raise the temperature of 1.5 x 103 g of water from 45°F to 130.°F? The specific heat of water is 4.184 J/g·°C.
A)3.0 x 101 kJ
B)3.0 x 102 kJ
C)3.4 x 102 kJ
D)5.3 x 102 kJ
E)8.2 x 102 kJ
A)3.0 x 101 kJ
B)3.0 x 102 kJ
C)3.4 x 102 kJ
D)5.3 x 102 kJ
E)8.2 x 102 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
6
Chemical energy is
A)the energy stored within the structural units of chemical substances.
B)the energy associated with the random motion of atoms and molecules.
C)solar energy, i.e.energy that comes from the sun.
D)energy available by virtue of an object's position.
A)the energy stored within the structural units of chemical substances.
B)the energy associated with the random motion of atoms and molecules.
C)solar energy, i.e.energy that comes from the sun.
D)energy available by virtue of an object's position.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
7
Thermal energy is
A)the energy stored within the structural units of chemical substances.
B)the energy associated with the random motion of atoms and molecules.
C)solar energy, i.e.energy that comes from the sun.
D)energy available by virtue of an object's position.
A)the energy stored within the structural units of chemical substances.
B)the energy associated with the random motion of atoms and molecules.
C)solar energy, i.e.energy that comes from the sun.
D)energy available by virtue of an object's position.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
8
An exothermic reaction causes the surroundings to
A)increase in temperature
B)become acidic.
C)expand.
D)decrease in temperature.
E)release CO2.
A)increase in temperature
B)become acidic.
C)expand.
D)decrease in temperature.
E)release CO2.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
9
Calculate the amount of heat necessary to raise the temperature of 135.0 g of water from 50.4°F to 85.0°F.The specific heat of water = 4.184 J/g·°C.
A)1.1 kJ
B)10.9 kJ
C)16.6 kJ
D)19.5 kJ
E)48.0 kJ
A)1.1 kJ
B)10.9 kJ
C)16.6 kJ
D)19.5 kJ
E)48.0 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
10
If 10.6 moles of water at 35°C absorbs 12.30 kJ, what is the final temperature of the water? The specific heat of water is 4.184 J/g·°C.
A)15°C
B)20°C
C)35°C
D)50.°C
E)312°C
A)15°C
B)20°C
C)35°C
D)50.°C
E)312°C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
11
A 22.0 g block of copper at 45°C absorbs 2.50 kJ of heat.Given the specific heat of Cu is 0.385 J/g·°C what will be the final temperature of the Cu?
A)45°C
B)340.°C
C)295°C
D)30.°C
E)250.°C
A)45°C
B)340.°C
C)295°C
D)30.°C
E)250.°C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
12
Aluminum metal has a specific heat of 0.900 J/g·°C.Calculate the amount of heat required to raise the temperature of 10.5 moles of Al from 30.5 °C to 225°C.
A)1.84 kJ
B)2.41 kJ
C)65.1 kJ
D)49.6 kJ
E)57.3 kJ
A)1.84 kJ
B)2.41 kJ
C)65.1 kJ
D)49.6 kJ
E)57.3 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
13
Radiant energy is
A)the energy stored within the structural units of chemical substances.
B)the energy associated with the random motion of atoms and molecules.
C)solar energy, i.e.energy that comes from the sun.
D)energy available by virtue of an object's position.
A)the energy stored within the structural units of chemical substances.
B)the energy associated with the random motion of atoms and molecules.
C)solar energy, i.e.energy that comes from the sun.
D)energy available by virtue of an object's position.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
14
An endothermic reaction causes the surroundings to
A)warm up.
B)become acidic.
C)condense.
D)decrease in temperature.
E)release CO2.
A)warm up.
B)become acidic.
C)condense.
D)decrease in temperature.
E)release CO2.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
15
A beaker contains 115 g of ethanol at 18.2°C.If the ethanol absorbs 1125 J of heat without losing heat to the surroundings, what will be the final temperature of the ethanol? The specific heat of ethanol is 2.46 J/g.°C.
A)4.08°C
B)14.1°C
C)18.4°C
D)22.2°C
E)36.4°C
A)4.08°C
B)14.1°C
C)18.4°C
D)22.2°C
E)36.4°C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
16
The specific heat of gold is 0.129 J/g·°C.What is the molar heat capacity of gold?
A)0.039 J/mol·°C
B)0.129 J/mol·°C
C)25.4 J/mol·°C
D)39.0 kJ/mol·°C
E)197 J/mol·°C
A)0.039 J/mol·°C
B)0.129 J/mol·°C
C)25.4 J/mol·°C
D)39.0 kJ/mol·°C
E)197 J/mol·°C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
17
A piece of copper with a mass of 218 g has a heat capacity of 83.9 J/°C.What is the specific heat of copper?
A)0.385 J/g·°C
B)1.32 J/g·°C
C)2.60 J/g·°C
D)24.5 J/g·°C
E)1.83 × 104 J/g·°C
A)0.385 J/g·°C
B)1.32 J/g·°C
C)2.60 J/g·°C
D)24.5 J/g·°C
E)1.83 × 104 J/g·°C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
18
Potential energy is
A)the energy stored within the structural units of chemical substances.
B)the energy associated with the random motion of atoms and molecules.
C)solar energy, i.e.energy that comes from the sun.
D)energy available by virtue of an object's position.
A)the energy stored within the structural units of chemical substances.
B)the energy associated with the random motion of atoms and molecules.
C)solar energy, i.e.energy that comes from the sun.
D)energy available by virtue of an object's position.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
19
A 135 g sample of H2O at 85°C is cooled.The water loses a total of 15 kJ of energy in the cooling process.What is the final temperature of the water? The specific heat of water is 4.184 J/g·°C.
A)27°C
B)58°C
C)70°C
D)84°C
E)112°C
A)27°C
B)58°C
C)70°C
D)84°C
E)112°C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
20
Given the specific heat for aluminum is 0.900 J/g·°C, how much heat is released when a 3.8 g sample of Al cools from 450.0°C to 25°C.
A)54 J
B)60 J
C)86 J
D)1.5 kJ
E)1.7 kJ
A)54 J
B)60 J
C)86 J
D)1.5 kJ
E)1.7 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
21
A 0.1326 g sample of magnesium was burned in an oxygen bomb calorimeter.The total heat capacity of the calorimeter plus water was 5,760 J/°C.If the temperature rise of the calorimeter with water was 0.570°C, calculate the enthalpy of combustion of magnesium. Mg(s)+ 1/2O2(g) MgO(s)
A)-3280 kJ/mol
B)-602 kJ/mol
C)-24.8 kJ/mol
D)106 kJ/mol
E)435 kJ/mol
A)-3280 kJ/mol
B)-602 kJ/mol
C)-24.8 kJ/mol
D)106 kJ/mol
E)435 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following has a H°f = 0 kJ/mol?
A)NO(g)
B)CS2(l)
C)Fe2+(aq)
D)H2O(l)
E)N2(g)
A)NO(g)
B)CS2(l)
C)Fe2+(aq)
D)H2O(l)
E)N2(g)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
23
Acetylene (C2H2)undergoes combustion in excess oxygen to generate gaseous carbon dioxide and water.Given H°f[CO2(g)] = -393.5 kJ/mol, H°f[H2O(g)] = -241.8 kJ/mol, and H°f [C2H2(g)] = 226.6 kJ/mol, how much energy is released (kJ)when 10.5 moles of acetylene is burned?
A)2,510.8 kJ
B)26,400 kJ
C)13,200 kJ
D)52,700 kJ
E)9,050 kJ
A)2,510.8 kJ
B)26,400 kJ
C)13,200 kJ
D)52,700 kJ
E)9,050 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
24
Naphthalene combustion can be used to calibrate the heat capacity of a bomb calorimeter.The heat of combustion of naphthalene is -40.1 kJ/g.When 0.8210 g of naphthalene was burned in a calorimeter containing 1,000.g of water, a temperature rise of 4.21°C was observed.What is the heat capacity of the bomb calorimeter excluding the water?
A)1.76 kJ/°C
B)3.64 kJ/°C
C)7.8 kJ/°C
D)15.3 kJ/°C
E)32.9 kJ/°
A)1.76 kJ/°C
B)3.64 kJ/°C
C)7.8 kJ/°C
D)15.3 kJ/°C
E)32.9 kJ/°

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
25
Find the standard enthalpy of formation of ethylene, C2H4(g), given the following data: heat of combustion of C2H4(g) = -1411 kJ/mol; H°f[CO2(g)] = -393.5 kJ/mol; H°f[H2O(l)] = -285.8 kJ/mol.
A)52 kJ/mol
B)87 kJ/mol
C)731 kJ/mol
D)1.41 × 103 kJ/mol
E)2.77 × 103 kJ/mol
A)52 kJ/mol
B)87 kJ/mol
C)731 kJ/mol
D)1.41 × 103 kJ/mol
E)2.77 × 103 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
26
When 18.5 g of HgO(s)is decomposed to form Hg(l)and O2(g), 7.75 kJ of heat is absorbed at standard-state conditions.What is the standard enthalpy of formation ( H°f)of HgO(s)?
A)-90.7 kJ/mol
B)-7.75 kJ/mol
C)0.419 kJ/mol
D)27.9 kJ/mol
E)143 kJ/mol
A)-90.7 kJ/mol
B)-7.75 kJ/mol
C)0.419 kJ/mol
D)27.9 kJ/mol
E)143 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
27
Octane (C8H18)undergoes combustion according to the following thermochemical equation: 2C8H18(l)+ 25O2(g) 16CO2(g)+ 18H2O(l)
H°rxn = -11,020 kJ/mol.
Given that H°f[CO2(g)] = -393.5 kJ/mol and H°f[H2O(l)] = -285.8 kJ/mol, calculate the standard enthalpy of formation of octane.
A)-210 kJ/mol
B)-11,230 kJ/mol
C)22,040 kJ/mol
D)-420 kJ/mol
E)420 kJ/mol
H°rxn = -11,020 kJ/mol.
Given that H°f[CO2(g)] = -393.5 kJ/mol and H°f[H2O(l)] = -285.8 kJ/mol, calculate the standard enthalpy of formation of octane.
A)-210 kJ/mol
B)-11,230 kJ/mol
C)22,040 kJ/mol
D)-420 kJ/mol
E)420 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
28
The reaction that represents the standard enthalpy of formation for acetone (CH3COCH3), a common ingredient in nail polish remover is:
A)3 C(graphite)+ 3 H2(g)+ ½ O2(g) CH3COCH3(l)
B)6 C(diamond)+ 6 H2(g)+ O2(g) 2 CH3COCH3(l)
C)3 C(diamond)+ 3 H2(g)+ ½ O2(g) CH3COCH3(l)
D)CH3COCH3(l) 3 C(graphite)+ 3 H2(g)+ ½ O2(g)
E)CH3COCH3(l)+ 4 O2(g) 3 CO2(g)+ 3 H2O(g)
A)3 C(graphite)+ 3 H2(g)+ ½ O2(g) CH3COCH3(l)
B)6 C(diamond)+ 6 H2(g)+ O2(g) 2 CH3COCH3(l)
C)3 C(diamond)+ 3 H2(g)+ ½ O2(g) CH3COCH3(l)
D)CH3COCH3(l) 3 C(graphite)+ 3 H2(g)+ ½ O2(g)
E)CH3COCH3(l)+ 4 O2(g) 3 CO2(g)+ 3 H2O(g)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
29
Butane (C4H10)undergoes combustion in excess oxygen to generate gaseous carbon dioxide and water.Given H°f [C4H10(g)] = -124.7 kJ/mol, H°f[CO2(g)] = -393.5 kJ/mol, H°f[H2O(g)] = -241.8 kJ/mol, how much energy is released (kJ)when 8.30 g of butane is burned?
A)22,100 kJ
B)2,658.3 kJ
C)379 kJ
D)759 kJ
E)2,910 kJ
A)22,100 kJ
B)2,658.3 kJ
C)379 kJ
D)759 kJ
E)2,910 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
30
The reaction that represents the standard enthalpy of formation for benzene (C6H6)is:
A)6 C(diamond)+ 3 H2(g) C6H6(l)
B)6 C(graphite)+ 6 H(g) C6H6(l)
C)C6H6(l)+ 15/2 O2(g) 6 CO2(g)+ 3 H2O(g)
D)6 C(graphite)+ 3 H2(g) C6H6(l)
E)C6H6(l) 6 C(graphite)+ 3 H2(g)
A)6 C(diamond)+ 3 H2(g) C6H6(l)
B)6 C(graphite)+ 6 H(g) C6H6(l)
C)C6H6(l)+ 15/2 O2(g) 6 CO2(g)+ 3 H2O(g)
D)6 C(graphite)+ 3 H2(g) C6H6(l)
E)C6H6(l) 6 C(graphite)+ 3 H2(g)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
31
Ethanol undergoes combustion in oxygen to produce carbon dioxide gas and liquid water.The standard heat of combustion of ethanol, C2H5OH(l), is -1366.8 kJ/mol.Given that H°f[CO2(g)] = -393.5 kJ/mol and H°f[H2O(l)] = -285.8 kJ/mol, what is the standard enthalpy of formation of ethanol?
A)-687.6 kJ/mol
B)-277.6 kJ/mol
C)687.6 kJ/mol
D)1,367 kJ/mol
E)3,010 kJ/mol
A)-687.6 kJ/mol
B)-277.6 kJ/mol
C)687.6 kJ/mol
D)1,367 kJ/mol
E)3,010 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
32
Glycine, C2H5O2N, is important for biological energy.The combustion reaction of glycine is given by the equation 4C2H5O2N(s)+ 9O2(g) 8CO2(g)+ 10H2O(l)+ 2N2(g) H°rxn = -3857 kJ/mol
Given that H°f[CO2(g)] = -393.5 kJ/mol and H°f[H2O(l)] = -285.8 kJ/mol, calculate the enthalpy of formation of glycine.
A)-3,178 kJ/mol
B)-964 kJ/mol
C)-537.2 kJ/mol
D)-268.2 kJ/mol
E)2,149 kJ/mol
Given that H°f[CO2(g)] = -393.5 kJ/mol and H°f[H2O(l)] = -285.8 kJ/mol, calculate the enthalpy of formation of glycine.
A)-3,178 kJ/mol
B)-964 kJ/mol
C)-537.2 kJ/mol
D)-268.2 kJ/mol
E)2,149 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following processes is exothermic, given the following:
N2(g)+ 2 O2(g) N2O4(l) H° = 9.67 kJ/mol
N2(g)+ 2 O2(g) 2 NO2(g) H° = 67.70 kJ/mol
A)2 N2(g)+ 4 O2(g) 2 N2O4(l)
B)½ N2(g)+ O2(g) ½ N2O4(l)
C)N2O4(l) N2(g)+ 2 O2(g)
D)2 N2(g)+ 4 O2(g) 2 NO2(g)+ N2O4(l)
E)2 N2(g)+ 4 O2(g) 4 NO2(g)
N2(g)+ 2 O2(g) N2O4(l) H° = 9.67 kJ/mol
N2(g)+ 2 O2(g) 2 NO2(g) H° = 67.70 kJ/mol
A)2 N2(g)+ 4 O2(g) 2 N2O4(l)
B)½ N2(g)+ O2(g) ½ N2O4(l)
C)N2O4(l) N2(g)+ 2 O2(g)
D)2 N2(g)+ 4 O2(g) 2 NO2(g)+ N2O4(l)
E)2 N2(g)+ 4 O2(g) 4 NO2(g)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
34
When 0.560 g of Na(s)reacts with excess F2(g)to form NaF(s), 13.8 kJ of heat is evolved at standard-state conditions.What is the standard enthalpy of formation ( H°f)of NaF(s)?
A)-570 kJ/mol
B)-24.8 kJ/mol
C)-7.8 kJ/mol
D)24.8 kJ/mol
E)570 kJ/mol
A)-570 kJ/mol
B)-24.8 kJ/mol
C)-7.8 kJ/mol
D)24.8 kJ/mol
E)570 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following processes is exothermic?
A)CH4(g)+ 2 O2(g) CO2(g)+ 2 H2O(l)
B)CO2(g)+ 2 H2O(l) CH4(g)+ 2 O2(g)
C)CO2(s) CO2(g)
D)H2O(l) H2O(g)
E)6 H2O(g)+ 4 CO2(g) 2 C2H6(g)+ 7 O2(g)
A)CH4(g)+ 2 O2(g) CO2(g)+ 2 H2O(l)
B)CO2(g)+ 2 H2O(l) CH4(g)+ 2 O2(g)
C)CO2(s) CO2(g)
D)H2O(l) H2O(g)
E)6 H2O(g)+ 4 CO2(g) 2 C2H6(g)+ 7 O2(g)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
36
A 100.mL sample of 0.200 M aqueous hydrochloric acid is added to 100.mL of 0.200 M aqueous ammonia in a calorimeter whose heat capacity (excluding any water)is 480.J/K.The following reaction occurs when the two solutions are mixed. HCl(aq)+ NH3(aq) NH4Cl(aq)
The temperature increase is 2.34°C.Calculate H per mole of HCl and NH3 reacted.
A)-154 kJ/mol
B)-1.96 kJ/mol
C)1.96 kJ/mol
D)154 kJ/mol
E)485 kJ/mol
The temperature increase is 2.34°C.Calculate H per mole of HCl and NH3 reacted.
A)-154 kJ/mol
B)-1.96 kJ/mol
C)1.96 kJ/mol
D)154 kJ/mol
E)485 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
37
The reaction that represents the standard enthalpy of formation for sucrose (C12H22O11)is:
A)C12H22O11(s)+ 12 O2 12 CO2(g)+ 11 H2O(g)
B)12 C(diamond)+ 11 H2(g)+ 11/2 O2(g) C12H22O11(s)
C)12 C(graphite)+ 11 H2(g)+ 11/2 O2(g) C12H22O11(s)
D)24 C(diamond)+ 22 H2(g)+ 11 O2(g) 2 C12H22O11(s)
E)C12H22O11(s) 12 C(graphite)+ 11 H2(g)+ 11/2 O2(g)
A)C12H22O11(s)+ 12 O2 12 CO2(g)+ 11 H2O(g)
B)12 C(diamond)+ 11 H2(g)+ 11/2 O2(g) C12H22O11(s)
C)12 C(graphite)+ 11 H2(g)+ 11/2 O2(g) C12H22O11(s)
D)24 C(diamond)+ 22 H2(g)+ 11 O2(g) 2 C12H22O11(s)
E)C12H22O11(s) 12 C(graphite)+ 11 H2(g)+ 11/2 O2(g)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following processes is endothermic?
A)O2(g)+ 2H2(g) 2H2O(g)
B)H2O(g) H2O(l)
C)3O2(g)+ 2CH3OH(g) 2CO2(g)+ 2H2O(g)
D)H2O(s) H2O(l)
A)O2(g)+ 2H2(g) 2H2O(g)
B)H2O(g) H2O(l)
C)3O2(g)+ 2CH3OH(g) 2CO2(g)+ 2H2O(g)
D)H2O(s) H2O(l)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following has a H°f = 0 kJ/mol?
A)CO2(g)
B)O3(g)
C)Cl-(aq)
D)NH3(aq)
E)I2(s)
A)CO2(g)
B)O3(g)
C)Cl-(aq)
D)NH3(aq)
E)I2(s)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following processes is endothermic, given the following: S(s)+ O2(g) SO2(g) H = -299 kJ/mol
S(s)+ 3/2 O2(g) SO3(g) H = -395 kJ/mol
A)2 S(s)+ 2 O2(g) 2 SO2(g)
B)½ S(s)+ ½ O2(g) ½ SO2(g)
C)2 S(s)+ 5/2 O2(g) SO2(g)+ SO3(g)
D)SO3(g) S(s)+ 3/2 O2(g)
E)2 S(s)+ 3 O2(g)F 2 SO3(g)
S(s)+ 3/2 O2(g) SO3(g) H = -395 kJ/mol
A)2 S(s)+ 2 O2(g) 2 SO2(g)
B)½ S(s)+ ½ O2(g) ½ SO2(g)
C)2 S(s)+ 5/2 O2(g) SO2(g)+ SO3(g)
D)SO3(g) S(s)+ 3/2 O2(g)
E)2 S(s)+ 3 O2(g)F 2 SO3(g)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
41
Concerning the reaction 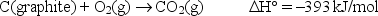 how many grams of C(graphite)must be burned to release 275 kJ of heat?
how many grams of C(graphite)must be burned to release 275 kJ of heat?
A)0.70 g
B)8.40 g
C)12.0 g
D)17.1 g
E)22.3 g
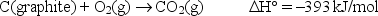 how many grams of C(graphite)must be burned to release 275 kJ of heat?
how many grams of C(graphite)must be burned to release 275 kJ of heat?A)0.70 g
B)8.40 g
C)12.0 g
D)17.1 g
E)22.3 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
42
An average home in Colorado requires 20.GJ of heat per month.How many grams of natural gas (methane)must be burned to supply this energy?
CH4(g)+ 2O2(g) CO2(g)+ 2H2O(l), H°rxn= -890.4 kJ/mol
A)7.1 × 10-4 g
B)1.4 × 103 g
C)1.4 × 104 g
D)2.2 × 104 g
E)3.6 × 105 g
CH4(g)+ 2O2(g) CO2(g)+ 2H2O(l), H°rxn= -890.4 kJ/mol
A)7.1 × 10-4 g
B)1.4 × 103 g
C)1.4 × 104 g
D)2.2 × 104 g
E)3.6 × 105 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
43
Calculate the standard enthalpy change for the reaction
2C8H18(l)+ 17O2(g) 16CO(g)+ 18H2O(l).
Given: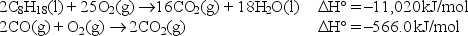
A)-10.450 kJ/mol
B)-6,492 kJ/mol
C)6,492 kJ/mol
D)10,450 kJ/mol
E)15,550 kJ/mol
2C8H18(l)+ 17O2(g) 16CO(g)+ 18H2O(l).
Given:
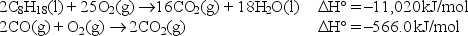
A)-10.450 kJ/mol
B)-6,492 kJ/mol
C)6,492 kJ/mol
D)10,450 kJ/mol
E)15,550 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
44
Given H2(g)+ (1/2)O2(g) H2O(l), H° = -286 kJ/mol, determine the standard enthalpy change for the reaction
2H2O(l) 2H2(g)+ O2(g).
A)( H° = -286 kJ/mol)
B)( H° = +286 kJ/mol)
C)( H° = -572 kJ/mol)
D)( H° = +572 kJ/mol)
E)( H° = -143 kJ/mol)
2H2O(l) 2H2(g)+ O2(g).
A)( H° = -286 kJ/mol)
B)( H° = +286 kJ/mol)
C)( H° = -572 kJ/mol)
D)( H° = +572 kJ/mol)
E)( H° = -143 kJ/mol)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
45
Calculate the standard enthalpy change for the reaction
2C8H18(l)+ 21O2(g) 8CO(g)+ 8CO2(g)+ 18H2O(l).
Given:
2C8H18(l)+ 25O2(g) 16CO2(g)+ 18H2O(l) H° = -11,020 kJ/mol
2CO(g)+ O2(g) 2CO2(g) H° = -566.0 kJ/mol
A)-1.0454 × 104 kJ/mol
B)-8,756 kJ/mol
C)-6,492 kJ/mol
D)1.0454 × 104 kJ/mol
E)1.1586 × 104 kJ/mol
2C8H18(l)+ 21O2(g) 8CO(g)+ 8CO2(g)+ 18H2O(l).
Given:
2C8H18(l)+ 25O2(g) 16CO2(g)+ 18H2O(l) H° = -11,020 kJ/mol
2CO(g)+ O2(g) 2CO2(g) H° = -566.0 kJ/mol
A)-1.0454 × 104 kJ/mol
B)-8,756 kJ/mol
C)-6,492 kJ/mol
D)1.0454 × 104 kJ/mol
E)1.1586 × 104 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
46
How much heat (kJ)is evolved when 4.50 g of Fe2O3 is reacted with excess carbon monoxide using the equation below?
Fe2O3(s)+ 3CO(g) 2 Fe(s)+ 3 CO2(g) , H°rxn = - 24.8 kJ/mol,
A)0.699 kJ
B)2.10 kJ
C)17.9 kJ
D)24.8 kJ
E)112 kJ
Fe2O3(s)+ 3CO(g) 2 Fe(s)+ 3 CO2(g) , H°rxn = - 24.8 kJ/mol,
A)0.699 kJ
B)2.10 kJ
C)17.9 kJ
D)24.8 kJ
E)112 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
47
The combustion of pentane produces heat according to the equation C5H12(l)+ 8O2(g) 5CO2(g)+ 6H2O(l), H°rxn= -3,510 kJ/mol
How many grams of CO2 are produced per 2.50 × 103 kJ of heat released?
A)0.0809 g
B)3.56 g
C)31.3 g
D)157 g
E)309 g
How many grams of CO2 are produced per 2.50 × 103 kJ of heat released?
A)0.0809 g
B)3.56 g
C)31.3 g
D)157 g
E)309 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
48
Calculate the heat required when 2.50 mol of A reacts with excess B and A2B according to the reaction: 2A + B + A2B 2AB + A2
Given:
A)10.0 kJ
B)12.5 kJ
C)25.0 kJ
D)35.0 kJ
E)62.5 kJ
Given:

A)10.0 kJ
B)12.5 kJ
C)25.0 kJ
D)35.0 kJ
E)62.5 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
49
Given 2Al(s)+ (3/2)O2(g) Al2O3(s), H°f = -1,670 kJ/mol for Al2O3 (s). Determine H° for the reaction 2Al2O3(s) 4Al(s)+ 3O2(g).
A)-3,340 kJ/mol
B)-1,670 kJ/mol
C)-835 kJ/mol
D)1,670 kJ/mol
E)3,340 kJ/mol
A)-3,340 kJ/mol
B)-1,670 kJ/mol
C)-835 kJ/mol
D)1,670 kJ/mol
E)3,340 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
50
The combustion of butane produces heat according to the equation 2C4H10(g)+ 13O2(g) 8CO2(g)+ 10H2O(l), H°rxn= -5,314 kJ/mol
How many grams of butane must be burned to release 1.00 × 104 kJ of heat?
A)30.9 g
B)61.8 g
C)109 g
D)153 g
E)219 g
How many grams of butane must be burned to release 1.00 × 104 kJ of heat?
A)30.9 g
B)61.8 g
C)109 g
D)153 g
E)219 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
51
Determine the heat given off to the surroundings when 9.0 g of aluminum reacts according to the equation 2Al + Fe2O3 Al2O3 + 2Fe, H°rxn= -849 kJ/mol.
A)1.4 × 102 kJ
B)2.8 × 102 kJ
C)5.6 × 102 kJ
D)2.5 × 103 kJ
E)7.6 × 103 kJ
A)1.4 × 102 kJ
B)2.8 × 102 kJ
C)5.6 × 102 kJ
D)2.5 × 103 kJ
E)7.6 × 103 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
52
Pentaborane B5H9(s)burns vigorously in O2 to give B2O3(s)and H2O(l).Calculate Hrxn for the combustion of 5.00 mol of B5H9.
H°f[B2O3(s)] = -1,273.5 kJ/mol
H°f[B5H9(s)] = 73.2 kJ/mol
H°f[H2O(l)] = -285.8 kJ/mol
A)- 45,400 kJ
B)45,400 kJ
C)- 22,700 kJ
D)- 9,090 kJ
E)- 8,790 kJ
H°f[B2O3(s)] = -1,273.5 kJ/mol
H°f[B5H9(s)] = 73.2 kJ/mol
H°f[H2O(l)] = -285.8 kJ/mol
A)- 45,400 kJ
B)45,400 kJ
C)- 22,700 kJ
D)- 9,090 kJ
E)- 8,790 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
53
Calculate the standard enthalpy of formation of liquid methanol, CH3OH(l), using the following information: 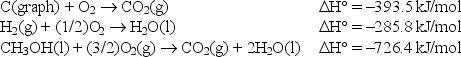
A)-1,691.5 kJ/mol
B)-238.7 kJ/mol
C)-47.1 kJ/mol
D)47.1 kJ/mol
E)1691.5 kJ/mol
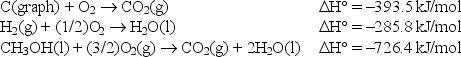
A)-1,691.5 kJ/mol
B)-238.7 kJ/mol
C)-47.1 kJ/mol
D)47.1 kJ/mol
E)1691.5 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
54
Calculate the standard enthalpy change for the reaction
2A + 2A2 + 4AB + B 5A2B
Given:
A)- 95.0 kJ/mol
B)- 60.0 kJ/mol
C)- 15.0 kJ/mol
D)10.0 kJ/mol
E)45.0 kJ/mol
2A + 2A2 + 4AB + B 5A2B
Given:

A)- 95.0 kJ/mol
B)- 60.0 kJ/mol
C)- 15.0 kJ/mol
D)10.0 kJ/mol
E)45.0 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
55
Calculate the standard enthalpy change for the reaction
4A + 2B 2AB + A2
Given:
A)- 95.0 kJ/mol
B)- 60.0 kJ/mol
C)- 15.0 kJ/mol
D)10.0 kJ/mol
E)45.0 kJ/mol
4A + 2B 2AB + A2
Given:

A)- 95.0 kJ/mol
B)- 60.0 kJ/mol
C)- 15.0 kJ/mol
D)10.0 kJ/mol
E)45.0 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
56
The combustion of butane produces heat according to the equation 2C4H10(g)+ 13O2(g) 8CO2(g)+ 10H2O(l), H°rxn= -5,314 kJ/mol
How many grams of CO2 are produced per 1.00 × 104 kJ of heat released?
A)23.4 g
B)44.0 g
C)82.3 g
D)187 g
E)662 g
How many grams of CO2 are produced per 1.00 × 104 kJ of heat released?
A)23.4 g
B)44.0 g
C)82.3 g
D)187 g
E)662 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
57
Given the thermochemical equation 2SO2(g)+ O2(g) 2SO3(g), H°rxn= -198 kJ/mol, how much heat is evolved when 600.g of SO2 is burned?
A)5.46 × 10-2 kJ
B)928 kJ
C)1.85 × 103 kJ
D)3.71 × 103 kJ
E)59,400 kJ
A)5.46 × 10-2 kJ
B)928 kJ
C)1.85 × 103 kJ
D)3.71 × 103 kJ
E)59,400 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
58
Styrene, C8H8, is one of the substances used in the production of synthetic rubber.When styrene burns in oxygen to form carbon dioxide and liquid water under standard-state conditions at 25°C, 42.62 kJ are released per gram of styrene.Find the standard enthalpy of formation of styrene at 25°C. (Given: H°f[CO2(g)] = -393.5 kJ/mol, H°f[H2O(l)] = -285.8 kJ/mol, H°f[H2O(g)] = -241.8 kJ/mol)
A)147.8 kJ/mol
B)323.8 kJ/mol
C)~636.7 kJ/mol
D)~4249 kJ/mol
E)~8730 kJ/mol
A)147.8 kJ/mol
B)323.8 kJ/mol
C)~636.7 kJ/mol
D)~4249 kJ/mol
E)~8730 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
59
During volcanic eruptions, hydrogen sulfide gas is given off and oxidized by air according to the following chemical equation:
2H2S(g)+ 3O2(g) 2SO2(g)+ 2H2O(g)
Calculate the standard enthalpy change for the above reaction given: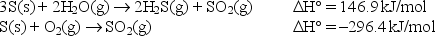
A)-1036.1 kJ/mol
B)-742.3 kJ/mol
C)-149.5 kJ/mol
D)443.3 kJ/mol
E)742.3 kJ/mol
2H2S(g)+ 3O2(g) 2SO2(g)+ 2H2O(g)
Calculate the standard enthalpy change for the above reaction given:
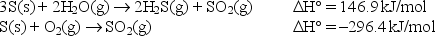
A)-1036.1 kJ/mol
B)-742.3 kJ/mol
C)-149.5 kJ/mol
D)443.3 kJ/mol
E)742.3 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
60
Given that CaO(s)+ H2O(l) Ca(OH)2(s), H°rxn = -64.8 kJ/mol, how many grams of CaO must react in order to liberate 525 kJ of heat?
A)6.92 g
B)56.1 g
C)455 g
D)606 g
E)3.40 × 104 g
A)6.92 g
B)56.1 g
C)455 g
D)606 g
E)3.40 × 104 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
61
A gas is allowed to expand, at constant temperature, from a volume of 1.0 L to 10.1 L against an external pressure of 0.50 atm.If the gas absorbs 250 J of heat from the surroundings, what are the values of q, w, and E? 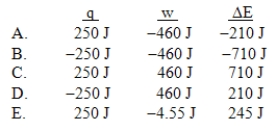
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
62
The enthalpy change when a strong acid is neutralized by strong base is -56.1 kJ/mol.If 135 mL of 0.450 M HI at 23.15°C is mixed with 145 mL of 0.500 M NaOH, also at 23.15°C, what will the maximum temperature reached by the resulting solution? (Assume that there is no heat loss to the container, that the specific heat of the final solution is 4.18 J/g·°C, and that the density of the final solution is that of water.)
A)20.24°C
B)26.06°C
C)29.19°C
D)32.35°C
E)36.57°C
A)20.24°C
B)26.06°C
C)29.19°C
D)32.35°C
E)36.57°C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
63
At 25°C, the following heats of reaction are known:
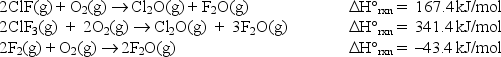
At the same temperature, use the above data to calculate the heat released (kJ)when 3.40 moles of ClF(g)reacts with excess F2: ClF(g)+ F2(g) ClF3(g)
A)109 kJ
B)233 kJ
C)370.kJ
D)465 kJ
E)1,580 kJ
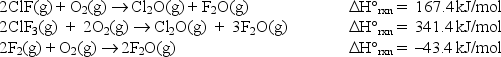
At the same temperature, use the above data to calculate the heat released (kJ)when 3.40 moles of ClF(g)reacts with excess F2: ClF(g)+ F2(g) ClF3(g)
A)109 kJ
B)233 kJ
C)370.kJ
D)465 kJ
E)1,580 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
64
Ethanol (C2H5OH)burns according to the equation C2H5OH(l)+ 3O2(g) 2CO2(g)+ 3H2O(l), H°rxn = -1367 kJ/mol.
How much heat is released when 35.0 g of ethanol is burned?
A)9.61 × 10-4 kJ
B)1,040 kJ
C)1,367 kJ
D)1,797 kJ
E)4.78 × 104 kJ
How much heat is released when 35.0 g of ethanol is burned?
A)9.61 × 10-4 kJ
B)1,040 kJ
C)1,367 kJ
D)1,797 kJ
E)4.78 × 104 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
65
Calcium oxide and water react in an exothermic reaction: CaO(s)+ H2O(l) Ca(OH)2(s), H°rxn = -64.8 kJ/mol
How much heat would be liberated when 7.15 g CaO(s)is dropped into a beaker containing 152g H2O?
A)1.97 × 10-3 kJ
B)8.26 kJ
C)508 kJ
D)547 kJ
E)555 kJ
How much heat would be liberated when 7.15 g CaO(s)is dropped into a beaker containing 152g H2O?
A)1.97 × 10-3 kJ
B)8.26 kJ
C)508 kJ
D)547 kJ
E)555 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
66
The heat of solution of LiCl is -37.1 kJ/mol, and the lattice energy of LiCl(s)is 828 kJ/mol.Calculate the total heat of hydration of 1.00 mol of gas phase Li+ ions and Cl- ions.
A)-865 kJ
B)-791 kJ
C)791 kJ
D)865 kJ
E)None of these.
A)-865 kJ
B)-791 kJ
C)791 kJ
D)865 kJ
E)None of these.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following processes always results in an increase in the energy of a system?
A)The system loses heat and does work on the surroundings.
B)The system gains heat and does work on the surroundings.
C)The system loses heat and has work done on it by the surroundings.
D)The system gains heat and has work done on it by the surroundings.
E)None of these is always true.
A)The system loses heat and does work on the surroundings.
B)The system gains heat and does work on the surroundings.
C)The system loses heat and has work done on it by the surroundings.
D)The system gains heat and has work done on it by the surroundings.
E)None of these is always true.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
68
The enthalpy change when a strong acid is neutralized by strong base is -56.1 kJ/mol.If 12.0 mL of 6.00 M HBr at 21.30°C is mixed with 300.mL of 0.250 M NaOH, also at 21.30°C, what will the maximum temperature reached by the resulting solution? (Assume that there is no heat loss to the container, that the specific heat of the final solution is 4.18 J/g·°C, and that the density of the final solution is that of water.)
A)18.20°C
B)24.40°C
C)24.53°C
D)34.25°C
E)101.8°C
A)18.20°C
B)24.40°C
C)24.53°C
D)34.25°C
E)101.8°C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
69
A gas is compressed in a cylinder from a volume of 20.0 L to 2.0 L by a constant pressure of 10.0 atm.Calculate the amount of work done on the system.
A)-1.81 × 104 J
B)-180 J
C)180 J
D)1.01 × 104 J
E)1.81 × 104 J
A)-1.81 × 104 J
B)-180 J
C)180 J
D)1.01 × 104 J
E)1.81 × 104 J

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
70
According to the first law of thermodynamics:
A)Energy is neither lost nor gained in any energy transformations.
B)Perpetual motion is possible.
C)Energy is conserved in quality but not in quantity.
D)Energy is being created as time passes.We have more energy in the universe now than when time began.
A)Energy is neither lost nor gained in any energy transformations.
B)Perpetual motion is possible.
C)Energy is conserved in quality but not in quantity.
D)Energy is being created as time passes.We have more energy in the universe now than when time began.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
71
The heat of solution of KCl is 17.2 kJ/mol and the lattice energy of KCl(s)is 701.2 kJ/mol.Calculate the total heat of hydration of 1.00 mol of gas phase K+ ions and Cl- ions.
A)-718 kJ
B)-684 kJ
C)684 kJ
D)718 kJ
E)None of these.
A)-718 kJ
B)-684 kJ
C)684 kJ
D)718 kJ
E)None of these.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
72
For which of these reactions will the difference between H° and E° be the smallest?
A)N2(g)+ 3H2(g) 2NH3(g)
B)4PH3(g) P4(g)+ 6H2(g)
C)H2(g)+ Cl2(g) 2HCl(g)
D)CO2(g)+ 2H2O(l) CH4(g)+ 2O2(g)
E)P4(s)+ 10Cl2(g) 4PCl5(s)
A)N2(g)+ 3H2(g) 2NH3(g)
B)4PH3(g) P4(g)+ 6H2(g)
C)H2(g)+ Cl2(g) 2HCl(g)
D)CO2(g)+ 2H2O(l) CH4(g)+ 2O2(g)
E)P4(s)+ 10Cl2(g) 4PCl5(s)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
73
For which of these reactions will the difference between H° and E° be the greatest?
A)2H2O2(l) 2H2O(l)+ O2(g)
B)CaCO3(s) CaO(s)+CO2(g)
C)NO(g)+ O3(g) NO2(g)+ O2(g)
D)2C2H6(g)+ 7O2(g) 4CO2(g)+ 6H2O(l)
E)4NH3(g)+ 5O2(g) 4NO(g)+ 6H2O(g)
A)2H2O2(l) 2H2O(l)+ O2(g)
B)CaCO3(s) CaO(s)+CO2(g)
C)NO(g)+ O3(g) NO2(g)+ O2(g)
D)2C2H6(g)+ 7O2(g) 4CO2(g)+ 6H2O(l)
E)4NH3(g)+ 5O2(g) 4NO(g)+ 6H2O(g)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
74
Calculate the amount of work done, in joules, when 2.5 mole of H2O vaporizes at 1.0 atm and 25°C.Assume the volume of liquid H2O is negligible compared to that of vapor.(1 L·atm = 101.3 J)
A)61.1 J
B)518 J
C)5.66 kJ
D)6.19 kJ
E)6,190 kJ
A)61.1 J
B)518 J
C)5.66 kJ
D)6.19 kJ
E)6,190 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
75
Solid sodium peroxide (Na2O2)reacts with liquid water yielding aqueous sodium hydroxide and oxygen gas.How much heat is released when 250.0 L of oxygen gas is produced from the reaction of sodium peroxide and water if the reaction is carried out in an open container at 1.000 atm pressure and 25°C? (Given: H°f[Na2O2(s)] = -510.9 kJ/mol; H°f[NaOH(aq)] = -469.2 kJ/mol; H°f[H2O(l)] = -285.8 kJ/mol)
A)141.7 kJ
B)1740 kJ
C)2900 kJ
D)3330 kJ
E)35,400 kJ
A)141.7 kJ
B)1740 kJ
C)2900 kJ
D)3330 kJ
E)35,400 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
76
At 25°C, the standard enthalpy of formation of anhydrous sodium carbonate is -1130.9 kJ/mol, whereas the standard enthalpy of formation of sodium carbonate monohydrate is -1430.1 kJ/mol.Determine H° at 25°C for the reaction Na2CO3(s)+ H2O(l) Na2CO3·H2O(s).
(Given: H°f[H2O(l)] = -285.8 kJ/mol)
A)-585.0 kJ/mol
B)-299.2 kJ/mol
C)-285.8 kJ/mol
D)-156.3 kJ/mol
E)-13.4 kJ/mol
(Given: H°f[H2O(l)] = -285.8 kJ/mol)
A)-585.0 kJ/mol
B)-299.2 kJ/mol
C)-285.8 kJ/mol
D)-156.3 kJ/mol
E)-13.4 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
77
Calculate the amount of work done against an atmospheric pressure of 1.00 atm when 500.0 g of zinc dissolves in excess acid at 30.0°C.
Zn(s)+ 2H+(aq) Zn2+(aq)+ H2(g)
A)w = +22.4 kJ
B)w = +24.9 kJ
C)w = 0
D)w = -2.52 kJ
E)w = -19.3 kJ
Zn(s)+ 2H+(aq) Zn2+(aq)+ H2(g)
A)w = +22.4 kJ
B)w = +24.9 kJ
C)w = 0
D)w = -2.52 kJ
E)w = -19.3 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
78
Find the heat absorbed from the surroundings when 15 g of O2 reacts according to the equation O + O2 O3, H°rxn= -103 kJ/mol.
A)4.6 × 10-3 kJ
B)32 kJ
C)48 kJ
D)96 kJ
E)110 kJ
A)4.6 × 10-3 kJ
B)32 kJ
C)48 kJ
D)96 kJ
E)110 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
79
Methanol (CH3OH)burns according to the equation 2CH3OH(l)+ 3O2(g) 2CO2(g)+ 4H2O(l), H°rxn = -1454 kJ/mol.
How much heat, in kilojoules, is given off when 75.0 g of methanol is burned?
A)727 kJ
B)3.22 × 103 kJ
C)1.45 × 103 kJ
D)1.70 × 103 kJ
E)3.41 × 103 kJ
How much heat, in kilojoules, is given off when 75.0 g of methanol is burned?
A)727 kJ
B)3.22 × 103 kJ
C)1.45 × 103 kJ
D)1.70 × 103 kJ
E)3.41 × 103 kJ

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
80
The total heat of hydration of 1.00 mol of gas phase Li+ ions and Cl - ions is -865 kJ.The lattice energy of LiCl(s)is 828 kJ/mol.Calculate the heat of solution of LiCl.
A)-1,693 kJ/mol
B)-37 kJ/mol
C)37 kJ/mol
D)1,693 kJ/mol
E)None of these
A)-1,693 kJ/mol
B)-37 kJ/mol
C)37 kJ/mol
D)1,693 kJ/mol
E)None of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck



