Deck 23: Transition Metal Chemistry and Coordination Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/92
Play
Full screen (f)
Deck 23: Transition Metal Chemistry and Coordination Compounds
1
Which of the following choices can function as a bidentate ligand?
A)PH3
B)Cl-
C)CN-
D)(-OCH2CH2CH2O-)
E)CO
A)PH3
B)Cl-
C)CN-
D)(-OCH2CH2CH2O-)
E)CO
(-OCH2CH2CH2O-)
2
The electron configuration of a Ti atom is
A)[Ne]3s23d2.
B)[Ne] 3s24d2.
C)[Ar]4s23d2.
D)[Ar]4s24d2.
E)[Ar]3d4.
A)[Ne]3s23d2.
B)[Ne] 3s24d2.
C)[Ar]4s23d2.
D)[Ar]4s24d2.
E)[Ar]3d4.
[Ar]4s23d2.
3
In [Cu(NH3)4]CO3, how many 3d electrons does copper have?
A)6
B)7
C)8
D)9
E)10
A)6
B)7
C)8
D)9
E)10
9
4
The total number of electrons in the 3d orbitals of Ti3+ is
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
5
How would you expect the molecule 1,10-phenanthroline (shown below)to function as a ligand? 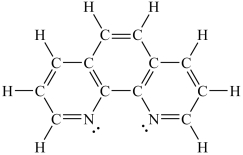
A)It would be expected to be a monodentate ligand.
B)It would be expected to be a bidentate ligand.
C)It would be expected to be a tridentate ligand.
D)It would be expected to be a tetradentate ligand.
E)It would not be expected to function as a ligand.

A)It would be expected to be a monodentate ligand.
B)It would be expected to be a bidentate ligand.
C)It would be expected to be a tridentate ligand.
D)It would be expected to be a tetradentate ligand.
E)It would not be expected to function as a ligand.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
6
How many 3d electrons does a Mn2+ ion have?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
7
The electron configuration of a nickel atom is
A)[Ar]4s23d6.
B)[Ar]4s13d7.
C)[Ar]3d8.
D)[Ar]4s23d8.
E)[Ar]3d10.
A)[Ar]4s23d6.
B)[Ar]4s13d7.
C)[Ar]3d8.
D)[Ar]4s23d8.
E)[Ar]3d10.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
8
The electron configuration of a Co3+ ion is
A)[Ar]3d6.
B)[Ar]4s13d5.
C)[Ar] 4s23d4.
D)[Ar]3d5.
E)[Ar]3d4.
A)[Ar]3d6.
B)[Ar]4s13d5.
C)[Ar] 4s23d4.
D)[Ar]3d5.
E)[Ar]3d4.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
9
How many 3d electrons does a V3+ ion have?
A)6
B)5
C)4
D)3
E)2
A)6
B)5
C)4
D)3
E)2

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
10
The total number of electrons in the 3d orbitals of a titanium atom is
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
11
The electron configuration of a Cr3+ ion is
A)[Ar]3d5.
B)[Ar]4s13d2.
C)[Ar]3d3.
D)[Ar]4s13d5.
E)[Ar]4s23d4.
A)[Ar]3d5.
B)[Ar]4s13d2.
C)[Ar]3d3.
D)[Ar]4s13d5.
E)[Ar]4s23d4.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
12
The total number of electrons in the 3d orbitals of Co3+ is
A)4
B)5
C)6
D)7
E)10
A)4
B)5
C)6
D)7
E)10

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following choices can function as a monodentate ligand only?
A)(H2NCH2CH2NH2)
B)(-O2CCO2-
C)(H2O)
D)(-OCH2CH2CH2O-)
E)(H2PCH2CH2PH2)
A)(H2NCH2CH2NH2)
B)(-O2CCO2-
C)(H2O)
D)(-OCH2CH2CH2O-)
E)(H2PCH2CH2PH2)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
14
The total number of electrons in the 3d orbitals of a copper atom is
A)6
B)7
C)8
D)9
E)10
A)6
B)7
C)8
D)9
E)10

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
15
The electron configuration of an Fe2+ ion is
A)[Ar]4s24d4.
B)[Ar]4s23d6.
C)[Ar]3d3.
D)[Ar]3d5.
E)[Ar]3d6.
A)[Ar]4s24d4.
B)[Ar]4s23d6.
C)[Ar]3d3.
D)[Ar]3d5.
E)[Ar]3d6.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
16
Assuming a coordination complex is formed with Fe2+ and 1,10-phenanthroline (shown below), which of the following statements is true? 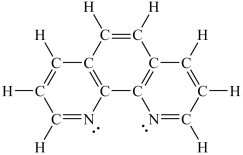
A)If two 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 2.
B)If two 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 6.
C)If three 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 3.
D)If three 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 6.
E)If four 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 4.

A)If two 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 2.
B)If two 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 6.
C)If three 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 3.
D)If three 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 6.
E)If four 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 4.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
17
In the complex ion [Fe(CN)6]4-, the oxidation number of Fe is
A)+1.
B)+2.
C)+3.
D)-4.
E)+6.
A)+1.
B)+2.
C)+3.
D)-4.
E)+6.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
18
In Na3[Ni(SCN)5], how many 3d electrons does nickel have?
A)6
B)7
C)8
D)9
E)10
A)6
B)7
C)8
D)9
E)10

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
19
In K4[Fe(CN)6], how many 3d electrons does the iron atom have?
A)3
B)4
C)5
D)6
E)7
A)3
B)4
C)5
D)6
E)7

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
20
The total number of electrons in the 3d orbitals of Cr3+ is
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
21
In the coordination compound [Cr(NH3)(en)2Cl]Br2, the coordination number (C.N.)and oxidation number (O.N.)of the metal atom are, respectively,
A)C.N.= 6; O.N.= +4.
B)C.N.= 6; O.N.= +3.
C)C.N.= 5; O.N.= +2.
D)C.N.= 4; O.N.= +2.
E)C.N.= 4; O.N.= +3.
A)C.N.= 6; O.N.= +4.
B)C.N.= 6; O.N.= +3.
C)C.N.= 5; O.N.= +2.
D)C.N.= 4; O.N.= +2.
E)C.N.= 4; O.N.= +3.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
22
In the coordination compound [Pt(NH3)2Cl2], the coordination number and oxidation number of the central atom are, respectively,
A)2, 0.
B)4, 4.
C)5, 0.
D)4, 2.
E)6, 2.
A)2, 0.
B)4, 4.
C)5, 0.
D)4, 2.
E)6, 2.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
23
In the complex ion [Co(en)2Br2]+, the oxidation number of Co is
A)+1.
B)+2.
C)+3.
D)-2.
E)-1.
A)+1.
B)+2.
C)+3.
D)-2.
E)-1.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
24
The best name for the complex shown below is 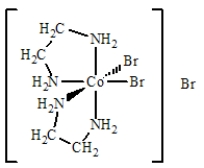
A)cobalt(III)bis(ethylenediamine)bromide.
B)dibromobis(ethylenediamine)cobalt(III)bromide.
C)dibromidedi(ethylenediamine)cobalt(III)bromide.
D)dibromodiethylenediaaminecobalt(III)bromide.
E)tribromobis(ethylenediamine)cobalt(III).

A)cobalt(III)bis(ethylenediamine)bromide.
B)dibromobis(ethylenediamine)cobalt(III)bromide.
C)dibromidedi(ethylenediamine)cobalt(III)bromide.
D)dibromodiethylenediaaminecobalt(III)bromide.
E)tribromobis(ethylenediamine)cobalt(III).

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
25
The correct formula for dicarbonylsilver (I)ion is:
A)Ag(CO)2
B)[Ag(CO)2]+
C)[Ag(CO)2]2+
D)[Ag(CO)2]-
E)[Ag(CO)2]2-
A)Ag(CO)2
B)[Ag(CO)2]+
C)[Ag(CO)2]2+
D)[Ag(CO)2]-
E)[Ag(CO)2]2-

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
26
In the coordination compound K2[Co(en)Cl4], the coordination number (C.N.)and oxidation number (O.N.)of cobalt are
A)C.N.= 6; O.N.= +2.
B)C.N.= 6; O.N.= +3.
C)C.N.= 5; O.N.= +2.
D)C.N.= 5; O.N.= +4.
E)C.N.= 4; O.N.= +3.
A)C.N.= 6; O.N.= +2.
B)C.N.= 6; O.N.= +3.
C)C.N.= 5; O.N.= +2.
D)C.N.= 5; O.N.= +4.
E)C.N.= 4; O.N.= +3.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
27
The correct name for Fe(CO)5 is:
A)pentacarbonyliron(V)
B)pentacarbonyliron(0)
C)hexacarbonyliron(0)
D)hexacarbonyliron(V)
E)pentacarbonateiron(0)
A)pentacarbonyliron(V)
B)pentacarbonyliron(0)
C)hexacarbonyliron(0)
D)hexacarbonyliron(V)
E)pentacarbonateiron(0)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
28
The correct formula for sodium tetracyanonickelate(II)ion is:
A)Ni(CN)4
B)[Ni(CN)4]+
C)[Ni(CN)4]2+
D)[Ni(CN)4]-
E)[Ni(CN)4]2-
A)Ni(CN)4
B)[Ni(CN)4]+
C)[Ni(CN)4]2+
D)[Ni(CN)4]-
E)[Ni(CN)4]2-

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
29
Give the coordination number (C.N.)and oxidation number (O.N.)of the metal atom in the coordination compound [Cr(NH3)2(en)Cl2].
A)C.N.= 4; O.N.= +2.
B)C.N.= 5; O.N.= +3.
C)C.N.= 5; O.N.= +2.
D)C.N.= 6; O.N.= +3.
E)C.N.= 6; O.N.= +2.
A)C.N.= 4; O.N.= +2.
B)C.N.= 5; O.N.= +3.
C)C.N.= 5; O.N.= +2.
D)C.N.= 6; O.N.= +3.
E)C.N.= 6; O.N.= +2.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
30
The correct formula for the dichlorobis(ethylenediamine)chromium(III)ion is
A)[Cr(en)2Cl2]3+.
B)[Cr(en)Cl2]+.
C)[Cr(en)2Cl2]2+.
D)[Cr(en)2Cl2]+.
E)[Cr(en)3Cl2]+.
A)[Cr(en)2Cl2]3+.
B)[Cr(en)Cl2]+.
C)[Cr(en)2Cl2]2+.
D)[Cr(en)2Cl2]+.
E)[Cr(en)3Cl2]+.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
31
In the coordination compound [Co(en)2Cl2]Cl, the coordination number and oxidation number of the central atom are, respectively,
A)4, +3.
B)6, +2.
C)4, +2.
D)6, +3.
E)4, +1.
A)4, +3.
B)6, +2.
C)4, +2.
D)6, +3.
E)4, +1.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
32
The best name for K4[FeCl2(CN)4] is
A)tetrapotassium dichlorodicyanoiron(II).
B)potassium dichlorodicyanoiron(II).
C)potassium dichlorodicyanoferrate(III).
D)tetrapotassium dichlorobis(cyano)iron(III).
E)potassium dichlorotetracyanoferrate(II).
A)tetrapotassium dichlorodicyanoiron(II).
B)potassium dichlorodicyanoiron(II).
C)potassium dichlorodicyanoferrate(III).
D)tetrapotassium dichlorobis(cyano)iron(III).
E)potassium dichlorotetracyanoferrate(II).

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
33
The correct formula for pentaamminechloroplatinum(IV)chloride is:
A)[Pt(NH3)5Cl]Cl3
B)[Pt(NH3)5Cl]Cl2
C)[Pt(NH3)5]Cl3
D)[Pt(NH3)5Cl]Cl4
E)[Pt(NH3)5]Cl4
A)[Pt(NH3)5Cl]Cl3
B)[Pt(NH3)5Cl]Cl2
C)[Pt(NH3)5]Cl3
D)[Pt(NH3)5Cl]Cl4
E)[Pt(NH3)5]Cl4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
34
Which of these octahedral complexes can have cis-trans isomers?
A)Co(NH3)3Cl3
B)[Co(NH3)5Cl]Cl2
C)[Co(NH3)6]Cl3
D)Na3[CoCl6]
E)Na2[Co(NH3)Cl5]
A)Co(NH3)3Cl3
B)[Co(NH3)5Cl]Cl2
C)[Co(NH3)6]Cl3
D)Na3[CoCl6]
E)Na2[Co(NH3)Cl5]

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
35
In the complex ion [Cr(C2O4)2(H2O)2]-, the oxidation number of Cr is
A)+1.
B)+2.
C)+3.
D)-2.
E)-1.
A)+1.
B)+2.
C)+3.
D)-2.
E)-1.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
36
Which of these square planar complex ions can have cis-trans isomers?
A)[Pt(NH3)4]2+
B)[Ni(NH3)4]2+
C)[Pt(NH3)2Cl2]
D)[Pt(NH3)Cl3]-
E)[Ni(NH3)3Cl]+
A)[Pt(NH3)4]2+
B)[Ni(NH3)4]2+
C)[Pt(NH3)2Cl2]
D)[Pt(NH3)Cl3]-
E)[Ni(NH3)3Cl]+

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
37
The correct name for [Fe(CO)6]Br3 is:
A)hexacarbonyliron(II)bromide
B)hexacarbonyliron(III)bromide
C)hexacarbonyltribromoiron(III)
D)hexacarbonyltribromoiron(II)
E)hexacarbonyliron(III)tribromide
A)hexacarbonyliron(II)bromide
B)hexacarbonyliron(III)bromide
C)hexacarbonyltribromoiron(III)
D)hexacarbonyltribromoiron(II)
E)hexacarbonyliron(III)tribromide

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
38
The coordination compound shown below has 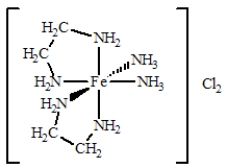
A)two monodentate ligands and two bidentate ligands.
B)three monodentate ligands and two bidentate ligands.
C)four monodentate ligands and two bidentate ligands.
D)one monodentate ligand and four bidentate ligands.
E)four bidentate ligands.

A)two monodentate ligands and two bidentate ligands.
B)three monodentate ligands and two bidentate ligands.
C)four monodentate ligands and two bidentate ligands.
D)one monodentate ligand and four bidentate ligands.
E)four bidentate ligands.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
39
Which response gives the correct coordination number (C.N.)and oxidation number (O.N.)of the transition metal atom in [Co(NH3)2(H2O)2Cl2]+?
A)C.N.= 2; O.N.= +3
B)C.N.= 3; O.N.= +1
C)C.N.= 4; O.N.= +2
D)C.N.= 6; O.N.= +1
E)C.N.= 6; O.N.= +3
A)C.N.= 2; O.N.= +3
B)C.N.= 3; O.N.= +1
C)C.N.= 4; O.N.= +2
D)C.N.= 6; O.N.= +1
E)C.N.= 6; O.N.= +3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
40
The correct name for K3[Fe(C2O4)3] is:
A)tripotassium trioxalatoiron(III)
B)potassium trioxalatoiron(II)
C)potassium trioxalatoiron(III)
D)tripotassium oxalatoiron(0)
E)potassium trioxalateiron(II)
A)tripotassium trioxalatoiron(III)
B)potassium trioxalatoiron(II)
C)potassium trioxalatoiron(III)
D)tripotassium oxalatoiron(0)
E)potassium trioxalateiron(II)

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
41
How many unpaired electrons are there in the complex ion [Mn(CN)6]3-?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
42
Which one of these complex ions would absorb light with the shortest wavelength?
A)[Co(H2O)6]2+
B)[Co(NH3)6]2+
C)[CoF6]4-
D)[Co(CN)6]4-
E)[Co(en)6]2+
A)[Co(H2O)6]2+
B)[Co(NH3)6]2+
C)[CoF6]4-
D)[Co(CN)6]4-
E)[Co(en)6]2+

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following complexes has optical (chiral)isomers?
A)[Co(NH3)4Br2]+
B)[Co(en)3]3+
C)Co(NH3)3Br3
D)[Co(en)Br4] -
E)[Co(en)(NH3)4] 3+
A)[Co(NH3)4Br2]+
B)[Co(en)3]3+
C)Co(NH3)3Br3
D)[Co(en)Br4] -
E)[Co(en)(NH3)4] 3+

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following complexes has optical (chiral)isomers?
A)[Co(H2O)4Br2]+
B)[Co(en)Cl4] -
C)[Co(en)(H2O)4] 3+
D)[Co(en)2Cl2]+
E)Co(H2O)3Cl3
A)[Co(H2O)4Br2]+
B)[Co(en)Cl4] -
C)[Co(en)(H2O)4] 3+
D)[Co(en)2Cl2]+
E)Co(H2O)3Cl3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
45
Which of these electron energy level patterns would absorb light with the shortest wavelength? 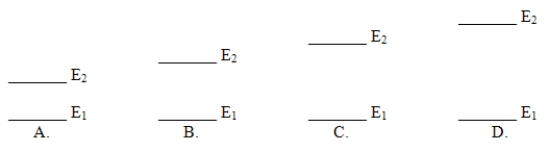
A)A
B)B
C)C
D)D

A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
46
Which of these complex ions would absorb light with the longest wavelength?
A)[Co(H2O)6]2+
B)[Co(NH3)6]2+
C)[CoF6]4-
D)[Co(CN)6]4-
E)[Co(en)6]2+
A)[Co(H2O)6]2+
B)[Co(NH3)6]2+
C)[CoF6]4-
D)[Co(CN)6]4-
E)[Co(en)6]2+

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
47
How many unpaired electrons does the manganese ion have in [Mn(CN)6]4-?
A)1 unpaired electron
B)2 unpaired electrons
C)3 unpaired electrons
D)5 unpaired electrons
E)No unpaired electrons
A)1 unpaired electron
B)2 unpaired electrons
C)3 unpaired electrons
D)5 unpaired electrons
E)No unpaired electrons

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
48
Two well-known complex ions containing Ni are [Ni(H2O)6]2+, which is green, and [Ni(en)3]2+, which is purple.Which one of these statements is true?
A)The crystal field splitting energy ( )is greater for [Ni(en)3]2+ than for [Ni(H2O)6]2+.
B)[Ni(en)3]2+ absorbs energy in the red region of the spectrum.
C)Both complex ions are diamagnetic.
D)[Ni(H2O)6]2+ transmits light with wavelengths of approximately 650-700 nm.
E)The green complex absorbs green light.
A)The crystal field splitting energy ( )is greater for [Ni(en)3]2+ than for [Ni(H2O)6]2+.
B)[Ni(en)3]2+ absorbs energy in the red region of the spectrum.
C)Both complex ions are diamagnetic.
D)[Ni(H2O)6]2+ transmits light with wavelengths of approximately 650-700 nm.
E)The green complex absorbs green light.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
49
In the complex ion [ML6]n+, Mn+ has seven d electrons and L is a weak field ligand.According to crystal field theory, the magnetic properties of the complex ion correspond to how many unpaired electrons?
A)0
B)1
C)2
D)3
E)5
A)0
B)1
C)2
D)3
E)5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
50
In the complex ion [ML6]n+, Mn+ has six d electrons and L is a weak field ligand.According to crystal field theory, the magnetic properties of the complex ion correspond to how many unpaired electrons?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
51
Which of these ligands produces the weakest crystal field?
A)CN-
B)I-
C)OH-
D)H2O
E)NH3
A)CN-
B)I-
C)OH-
D)H2O
E)NH3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
52
In the complex ion [ML6]n+, Mn+ has five d electrons and L is a strong field ligand.According to crystal field theory, the magnetic properties of the complex ion correspond to how many unpaired electrons?
A)0
B)1
C)2
D)3
E)5
A)0
B)1
C)2
D)3
E)5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
53
Labile complexes are coordination complexes that
A)are labeled with a radioactive isotope.
B)are thermodynamically stable.
C)are thermodynamically unstable.
D)undergo rapid ligand exchange reactions.
E)undergo very slow ligand exchange reactions.
A)are labeled with a radioactive isotope.
B)are thermodynamically stable.
C)are thermodynamically unstable.
D)undergo rapid ligand exchange reactions.
E)undergo very slow ligand exchange reactions.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
54
The number of geometrical isomers and optical isomers of the complex ion [Co(en)3]3+ are, respectively,
A)2 and 2.
B)1 and 1.
C)3 and 2.
D)1 and 2.
E)2 and 4.
A)2 and 2.
B)1 and 1.
C)3 and 2.
D)1 and 2.
E)2 and 4.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
55
Two complex ions containing Ni are [Ni(NH3)6]2+, which is blue, and [Ni(en)3]2+, which is purple.Which one of these statements is true?
A)The crystal field splitting energy ( )is greater for [Ni(NH3)6]2+ than for [Ni(en)3]2+.
B)[Ni(en)3]2+ absorbs light in the violet region of the spectrum.
C)Both complex ions are diamagnetic.
D)The energy of the photon absorbed by [Ni(en)3]2+ is greater than that absorbed by [Ni(NH3)6]2+.
E)The wavelength of the light absorbed by [Ni(en)3]2+ is greater than the wavelength absorbed by [Ni(NH3)6]2+.
A)The crystal field splitting energy ( )is greater for [Ni(NH3)6]2+ than for [Ni(en)3]2+.
B)[Ni(en)3]2+ absorbs light in the violet region of the spectrum.
C)Both complex ions are diamagnetic.
D)The energy of the photon absorbed by [Ni(en)3]2+ is greater than that absorbed by [Ni(NH3)6]2+.
E)The wavelength of the light absorbed by [Ni(en)3]2+ is greater than the wavelength absorbed by [Ni(NH3)6]2+.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
56
The ion [Co(NH3)6]2+ is octahedral and high spin.This complex is
A)paramagnetic, with 1 unpaired electron.
B)paramagnetic, with 3 unpaired electrons.
C)paramagnetic, with 4 unpaired electrons.
D)paramagnetic, with 5 unpaired electrons.
E)diamagnetic.
A)paramagnetic, with 1 unpaired electron.
B)paramagnetic, with 3 unpaired electrons.
C)paramagnetic, with 4 unpaired electrons.
D)paramagnetic, with 5 unpaired electrons.
E)diamagnetic.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
57
In the complex ion [ML6]n+, Mn+ has seven d electrons and L is a strong field ligand.According to crystal field theory, the magnetic properties of the complex ion correspond to how many unpaired electrons?
A)0
B)1
C)2
D)3
E)5
A)0
B)1
C)2
D)3
E)5

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
58
Which of these ligands produces the strongest crystal field?
A)Cl-
B)CO
C)OH-
D)H2O
E)NH3
A)Cl-
B)CO
C)OH-
D)H2O
E)NH3

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
59
Consider the [CoCl6]4- ion.Determine which responses are true statements. I.The oxidation number of Co is -4.
II.The coordination number of cobalt is 6.
III.It is paramagnetic.
IV.It is a low-spin complex.
A)I, II, III, IV
B)I, IV
C)II, III
D)I, III
E)III, IV
II.The coordination number of cobalt is 6.
III.It is paramagnetic.
IV.It is a low-spin complex.
A)I, II, III, IV
B)I, IV
C)II, III
D)I, III
E)III, IV

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
60
A complex with the composition [MA2B2]X2 is found to have no geometrical isomers.Both A and B are monodentate ligands.The structure of the complex is
A)linear.
B)square planar.
C)tetrahedral.
D)octahedral.
A)linear.
B)square planar.
C)tetrahedral.
D)octahedral.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
61
Write the chemical formula of triammineaquodichlorochromium(III)chloride.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
62
Write the chemical formula of the dibromobis(oxalato)cobaltate(III)ion.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
63
What is the oxidation number of cobalt in [Co(NH3)6]Cl3?

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
64
What is the oxidation number of Co in the complex Co(H2O)3Cl3?

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
65
Ethylenediaminetetraacetic acid (EDTA)is
A)not useful as a chelating agent.
B)an effective antidote for heavy metal poisoning (e.g., Pb2+ and Hg2+).
C)a monodentate ligand.
D)known to form unstable complex ions with Fe3+, Hg2+, and Zn2+.
E)known to form complexes with platinum that inhibit the growth of cancerous cells.
A)not useful as a chelating agent.
B)an effective antidote for heavy metal poisoning (e.g., Pb2+ and Hg2+).
C)a monodentate ligand.
D)known to form unstable complex ions with Fe3+, Hg2+, and Zn2+.
E)known to form complexes with platinum that inhibit the growth of cancerous cells.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
66
What is the oxidation number of Co in the complex Na[Co(H2O)2Cl4]?

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
67
A molecule or ion that provides an electron pair for coordinate covalent bond formation is called a Lewis ________.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
68
What terms describe the geometric isomers that are possible for the complex [CrF2Cl4]3-?

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
69
A molecule or atom that accepts an electron pair to form a coordinate covalent bond is called a Lewis _______.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
70
What is the oxidation number of Co in the complex [Co(H2O)4Cl2]Cl?

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
71
Name the complex ion [Ni(CN)4]2-.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
72
Draw and label the geometric isomers of [CrF2Cl4]3-?

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
73
What is the oxidation number of Fe in [Fe(CN)6]4-?

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
74
What is the coordination number of chromium in Cr(en)2Cl2?

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
75
Copper can be separated from iron in the ore chalcopyrite (CuFeS2)by heating the ore in the presence of oxygen to form copper(I)sulfide, iron(II)oxide, and sulfur dioxide.Write a balanced chemical equation for this process.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
76
What is the coordination number of cobalt in [Co(NH3)6]Cl3?

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
77
What is the coordination number of silver in [Ag(NH3)2]Cl?

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
78
How many geometric isomers are possible for the complex [CrF3Br3]3-? Draw these isomers.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
79
Cis-platinum complexes are
A)used in the extraction of silver and gold.
B)effective antidotes for heavy metal poisoning (e.g., Pb2+ and Hg2+).
C)used to provide nutrients for plants.
D)used to prevent eutrophication of lakes.
E)effective antitumor agents.
A)used in the extraction of silver and gold.
B)effective antidotes for heavy metal poisoning (e.g., Pb2+ and Hg2+).
C)used to provide nutrients for plants.
D)used to prevent eutrophication of lakes.
E)effective antitumor agents.

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck
80
Write the chemical formula of diamminedichloroplatinum(II).

Unlock Deck
Unlock for access to all 92 flashcards in this deck.
Unlock Deck
k this deck



