Deck 19: Electrochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/138
Play
Full screen (f)
Deck 19: Electrochemistry
1
Complete and balance the following redox equation. The sum of the smallest whole-number coefficients is Br2 BrO3- + Br- (basic solution)
A)9
B)12
C)18
D)21
E)None of the above.
A)9
B)12
C)18
D)21
E)None of the above.
18
2
Calculate E°cell for a silver-aluminum cell in which the cell reaction is Al(s)+ 3Ag+(aq) Al3+(aq)+ 3Ag(s)
A)-2.46 V
B)0.86 V
C)-0.86 V
D)2.46 V
E)none of these
A)-2.46 V
B)0.86 V
C)-0.86 V
D)2.46 V
E)none of these
2.46 V
3
Given the following notation for an electrochemical cell Pt(s)| H2(g)| H+(aq)|| Ag+(aq)| Ag(s)
What is the balanced overall (net)cell reaction?
A)2H+(aq)+ 2Ag+(aq) H2(g)+ 2Ag(s)
B)H2(g)+ 2Ag(s) H+(aq)+ 2Ag+(aq)
C)2H+(aq)+ 2Ag(s) H2(g)+ 2Ag+(aq)
D)H2(g)+ Ag+(aq) H+(aq)+ Ag(s)
E)H2(g)+ 2Ag+(aq) 2H+(aq)+ 2Ag(s)
What is the balanced overall (net)cell reaction?
A)2H+(aq)+ 2Ag+(aq) H2(g)+ 2Ag(s)
B)H2(g)+ 2Ag(s) H+(aq)+ 2Ag+(aq)
C)2H+(aq)+ 2Ag(s) H2(g)+ 2Ag+(aq)
D)H2(g)+ Ag+(aq) H+(aq)+ Ag(s)
E)H2(g)+ 2Ag+(aq) 2H+(aq)+ 2Ag(s)
H2(g)+ 2Ag+(aq) 2H+(aq)+ 2Ag(s)
4
Complete and balance the following redox equation using the smallest whole-number coefficients. What is the coefficient of Sn in the balanced equation? Sn + HNO3 SnO2 + NO2 + H2O (acidic solution)
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
5
Consider an electrochemical cell constructed from the following half cells, linked by a KCl salt bridge. • a Fe electrode in 1.0 M FeCl2 solution
• a Sn electrode in 1.0 M Sn(NO3)2 solution
When the cell is running spontaneously, which choice includes only true statements and no false ones?
A)The tin electrode loses mass and the tin electrode is the cathode.
B)The tin electrode gains mass and the tin electrode is the cathode.
C)The iron electrode gains mass and the iron electrode is the anode.
D)The iron electrode loses mass and the iron electrode is the cathode.
E)The iron electrode gains mass and the iron electrode is the cathode.
• a Sn electrode in 1.0 M Sn(NO3)2 solution
When the cell is running spontaneously, which choice includes only true statements and no false ones?
A)The tin electrode loses mass and the tin electrode is the cathode.
B)The tin electrode gains mass and the tin electrode is the cathode.
C)The iron electrode gains mass and the iron electrode is the anode.
D)The iron electrode loses mass and the iron electrode is the cathode.
E)The iron electrode gains mass and the iron electrode is the cathode.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
6
A certain electrochemical cell has for its cell reaction: Zn + HgO ZnO + Hg
Which is the half-reaction occurring at the anode?
A)HgO + 2e- Hg + O2-
B)Zn2+ + 2e- Zn
C)Zn Zn2+ + 2e-
D)ZnO + 2e- Zn
Which is the half-reaction occurring at the anode?
A)HgO + 2e- Hg + O2-
B)Zn2+ + 2e- Zn
C)Zn Zn2+ + 2e-
D)ZnO + 2e- Zn

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
7
Complete and balance the following redox equation using the set of smallest whole-numbers coefficients. What is the sum of the coefficients? HI + HNO3 I2 + NO (acidic solution)
A)5
B)7
C)14
D)17
E)None of these.
A)5
B)7
C)14
D)17
E)None of these.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
8
Complete and balance the following redox equation using the set of smallest whole-number coefficients. Now sum the coefficients of all species in the balanced equation. (Remember the coefficients that are equal to one.)The sum of the coefficients is BrO3-(aq)+ Sb3+(aq) Br-(aq)+ Sb5+(aq)(acidic solution)
A)4
B)12
C)13
D)17
E)None of these.
A)4
B)12
C)13
D)17
E)None of these.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
9
Consider an electrochemical cell constructed from the following half cells, linked by a KCl salt bridge. • a Fe electrode in 1.0 M FeCl2 solution
• a Ni electrode in 1.0 M Ni(NO3)2 solution
When the cell is running spontaneously, which choice includes only true statements and no false ones?
A)The nickel electrode loses mass and the nickel electrode is the cathode.
B)The nickel electrode gains mass and the nickel electrode is the cathode.
C)The iron electrode gains mass and the iron electrode is the anode.
D)The iron electrode loses mass and the iron electrode is the cathode.
• a Ni electrode in 1.0 M Ni(NO3)2 solution
When the cell is running spontaneously, which choice includes only true statements and no false ones?
A)The nickel electrode loses mass and the nickel electrode is the cathode.
B)The nickel electrode gains mass and the nickel electrode is the cathode.
C)The iron electrode gains mass and the iron electrode is the anode.
D)The iron electrode loses mass and the iron electrode is the cathode.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
10
Complete and balance the following redox equation. The sum of the smallest whole-number coefficients is MnO4- + H+ + Br- Mn2+ + Br2 + H2O (acidic solution)
A)6
B)17
C)21
D)29
E)43
A)6
B)17
C)21
D)29
E)43

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
11
Complete and balance the following redox equation. What is the coefficient of OH- when the equation is balanced using the set of smallest whole-number coefficients? MnO4- + I- MnO2 + IO3- (basic solution)
A)1
B)2
C)4
D)10
E)None of these.
A)1
B)2
C)4
D)10
E)None of these.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
12
Complete and balance the following redox equation. What is the coefficient of H2O when the equation is balanced using the set of smallest whole-number coefficients? MnO4- + SO32- Mn2+ + SO42- (acidic solution)
A)3
B)4
C)5
D)8
E)None of these.
A)3
B)4
C)5
D)8
E)None of these.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
13
Complete and balance the following redox equation. What is the coefficient of H2O when the equation is balanced with the set of smallest whole-number coefficients? H2O + MnO4- + I- MnO2 + IO3- (basic solution)
A)1
B)2
C)4
D)10
E)None of these.
A)1
B)2
C)4
D)10
E)None of these.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
14
Consider an electrochemical cell constructed from the following half cells, linked by an external circuit and by a KCl salt bridge. • an Al(s)electrode in 1.0 M Al(NO3)3 solution
• a Pb(s)electrode in 1.0 M Pb(NO3)2 solution
The balanced overall (net)cell reaction is
A)Pb(s)+ Al3+(aq) Pb2+(aq)+ Al(s.)
B)3Pb(s)+ 2Al3+(aq) 3Pb2+(aq)+ 2Al(s).
C)3Pb2+(aq)+ 2Al(s) 3Pb(s)+ 2Al3+(aq).
D)Pb2+(aq)+ Al(s) Pb(s)+ Al3+(aq).
• a Pb(s)electrode in 1.0 M Pb(NO3)2 solution
The balanced overall (net)cell reaction is
A)Pb(s)+ Al3+(aq) Pb2+(aq)+ Al(s.)
B)3Pb(s)+ 2Al3+(aq) 3Pb2+(aq)+ 2Al(s).
C)3Pb2+(aq)+ 2Al(s) 3Pb(s)+ 2Al3+(aq).
D)Pb2+(aq)+ Al(s) Pb(s)+ Al3+(aq).

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
15
Calculate the value of E°cell for the following reaction: 2Au(s)+ 3Ca2+(aq) 2Au3+(aq)+ 3Ca(s)
A)-4.37 V
B)-1.37 V
C)-11.6 V
D)1.37 V
E)4.37 V
A)-4.37 V
B)-1.37 V
C)-11.6 V
D)1.37 V
E)4.37 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
16
Complete and balance the following redox equation. What is the coefficient of H2S when the equation is balanced using the set of smallest whole-number coefficients? H2S + MnO4- Mn2+ + SO42- (acidic solution)
A)1
B)2
C)4
D)5
E)None of these.
A)1
B)2
C)4
D)5
E)None of these.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
17
Complete and balance the following redox equation. The sum of the smallest whole-number coefficients is Bi(OH)3 + SnO22- Bi + SnO32- (basic solution)
A)32
B)25
C)16
D)13
E)None of these.
A)32
B)25
C)16
D)13
E)None of these.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
18
Complete and balance the following redox equation. When properly balanced using the smallest whole-number coefficients, the coefficient of S is H2S + HNO3 S + NO (acidic solution)
A)1
B)2
C)3
D)5
E)6
A)1
B)2
C)3
D)5
E)6

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
19
Complete and balance the following redox equation that occurs in acidic solution using the set of smallest whole-number coefficients. What is the sum of all the coefficients in the equation? PbO2(s)+ Cl- Pb2+ + Cl2(g)(acidic solution)
A)2
B)4
C)5
D)9
E)11
A)2
B)4
C)5
D)9
E)11

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
20
Consider an electrochemical cell constructed from the following half cells, linked by a KCl salt bridge. • an Al(s)electrode in 0.5 M Al2(SO4)3 solution
• a Pb(s)electrode in 1.0 M Pb(NO3)2 solution
Which electrode is the anode?
A)Al
B)Pb
C)Neither
• a Pb(s)electrode in 1.0 M Pb(NO3)2 solution
Which electrode is the anode?
A)Al
B)Pb
C)Neither

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
21
Using a table of standard electrode potentials, decide which of the following statements is completely true.
A)Cu2+ can oxidize H2, and Fe can reduce Mn2+.
B)Ni2+ can oxidize Cu2+, and Fe2+ can reduce H+.
C)Fe2+ can oxidize H2, and Fe2+ can reduce Au3+.
D)Br2 can oxidize Ni, and H2 can reduce Mn2+.
E)H+ can oxidize Fe, and Ni can reduce Br2.
A)Cu2+ can oxidize H2, and Fe can reduce Mn2+.
B)Ni2+ can oxidize Cu2+, and Fe2+ can reduce H+.
C)Fe2+ can oxidize H2, and Fe2+ can reduce Au3+.
D)Br2 can oxidize Ni, and H2 can reduce Mn2+.
E)H+ can oxidize Fe, and Ni can reduce Br2.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
22
For the reaction, 2Cr2+ + Cl2(g) 2Cr3+ + 2Cl-, E°cell is 1.78 V.Calculate E°cell for the related reaction Cr3+ + Cl- Cr2+ + 1/2Cl2(g).
A)1.78 V
B)0.89 V
C)-1.78 V
D)-0.89 V
E)None of these.
A)1.78 V
B)0.89 V
C)-1.78 V
D)-0.89 V
E)None of these.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
23
For the reaction Ni2+(aq)+ 2Fe2+(aq) Ni(s)+ 2Fe3+(aq), the standard cell potential E°cell is
A)+2.81 V.
B)+1.02 V.
C)+0.52 V.
D)-1.02 V.
E)-2.81 V.
A)+2.81 V.
B)+1.02 V.
C)+0.52 V.
D)-1.02 V.
E)-2.81 V.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
24
Which one of the following reactions will occur spontaneously at standard-state conditions and 25°C?
A)Mg2+ + Ca Mg + Ca2+
B)Au + 3K+ Au3+ + 3K
C)2Al3+ + 3Fe 2Al + 3Fe2+
D)Cu + 2H+ Cu2+ + H2
A)Mg2+ + Ca Mg + Ca2+
B)Au + 3K+ Au3+ + 3K
C)2Al3+ + 3Fe 2Al + 3Fe2+
D)Cu + 2H+ Cu2+ + H2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
25
Consider a voltaic cell based on the following cell reaction: Ni(s)+ At2(s) Ni2+(aq)+ 2At- (aq)
Given that the standard cell emf is 0.55 V, what is the standard reduction potential for astatine? [E°(Ni2+/Ni)= -0.25 V]
A)0.80 V
B)0.30 V
C)-0.30 V
D)-0.80 V
E)0.43 V
Given that the standard cell emf is 0.55 V, what is the standard reduction potential for astatine? [E°(Ni2+/Ni)= -0.25 V]
A)0.80 V
B)0.30 V
C)-0.30 V
D)-0.80 V
E)0.43 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the following standard reduction potentials in acid solution: 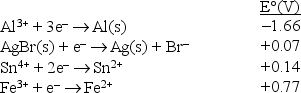 The strongest reducing agent among those shown above is
The strongest reducing agent among those shown above is
A)Fe3+.
B)Fe2+.
C)Br-.
D)Al3+.
E)Al.
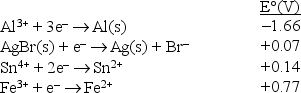 The strongest reducing agent among those shown above is
The strongest reducing agent among those shown above isA)Fe3+.
B)Fe2+.
C)Br-.
D)Al3+.
E)Al.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
27
The overall reaction 2Co3+(aq)+ 2Cl-(aq) 2Co2+(aq)+ Cl2(g)has the standard cell voltage E°cell= 0.46 V.Given E° = 1.36 V for the reaction Cl2(g)+ 2e- 2Cl-(aq), calculate the standard reduction potential for the following the half reaction at 25°C: Co3+ + e- Co2+
A)1.82 V
B)-0.90 V
C)0.90 V
D)-1.82 V
E)-1.36 V
A)1.82 V
B)-0.90 V
C)0.90 V
D)-1.82 V
E)-1.36 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
28
Consider the following standard reduction potentials in acid solution: 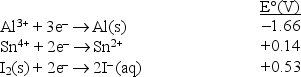 Which is the weakest oxidizing agent in this list?
Which is the weakest oxidizing agent in this list?
A)Al3+(aq)
B)Al(s)
C)I-(aq)
D)I2(s)
E)Sn4+(aq)
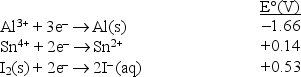 Which is the weakest oxidizing agent in this list?
Which is the weakest oxidizing agent in this list?A)Al3+(aq)
B)Al(s)
C)I-(aq)
D)I2(s)
E)Sn4+(aq)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
29
An electrochemical cell based on the following reaction has a standard cell voltage (E°cell)of 0.48 V: Sn(s)+ Cu2+(aq) Sn2+(aq)+ Cu(s)
What is the standard reduction potential of tin(II)? (E°(Cu2+/Cu)= 0.34 V)
A)-0.14 V
B)0.14 V
C)-0.82 V
D)0.82 V
E)none of these
What is the standard reduction potential of tin(II)? (E°(Cu2+/Cu)= 0.34 V)
A)-0.14 V
B)0.14 V
C)-0.82 V
D)0.82 V
E)none of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
30
Which statement is true for a spontaneous redox reaction carried out at standard-state conditions?
A)E°red is always negative.
B)E°cell is always positive.
C)E°ox is always positive.
D)E°red is always positive.
A)E°red is always negative.
B)E°cell is always positive.
C)E°ox is always positive.
D)E°red is always positive.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
31
According to the following cell diagram, which chemical species undergoes reduction? Sn | Sn2+ || NO3- (acid soln), NO(g)| Pt
A)Sn
B)Sn2+
C)NO3-
D)NO
E)Pt
A)Sn
B)Sn2+
C)NO3-
D)NO
E)Pt

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
32
Consider the following standard reduction potentials in acid solution: 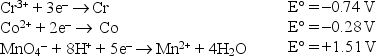 The strongest reducing agent listed above is
The strongest reducing agent listed above is
A)Cr3+.
B)Cr.
C)Mn2+.
D)Co.
E)MnO4-.
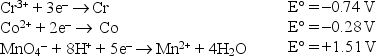 The strongest reducing agent listed above is
The strongest reducing agent listed above isA)Cr3+.
B)Cr.
C)Mn2+.
D)Co.
E)MnO4-.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
33
Calculate the standard cell emf for the following cell: Mg | Mg2+ || NO3- (acid soln)| NO(g)| Pt
A)3.33 V
B)1.41 V
C)-1.41 V
D)8.46 V
E)-8.46 V
A)3.33 V
B)1.41 V
C)-1.41 V
D)8.46 V
E)-8.46 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the following standard reduction potentials in acid solution: 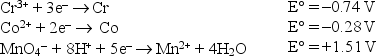 The strongest oxidizing agent listed above is
The strongest oxidizing agent listed above is
A)Cr3+.
B)Cr.
C)Mn2+.
D)Co2+.
E)MnO4-.
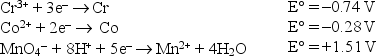 The strongest oxidizing agent listed above is
The strongest oxidizing agent listed above isA)Cr3+.
B)Cr.
C)Mn2+.
D)Co2+.
E)MnO4-.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
35
Consider the following standard reduction potentials in acid solution: 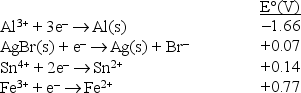 The strongest oxidizing agent among those shown above is
The strongest oxidizing agent among those shown above is
A)Fe3+.
B)Fe2+.
C)Br-.
D)Al3+.
E)Al.
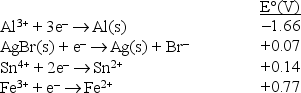 The strongest oxidizing agent among those shown above is
The strongest oxidizing agent among those shown above isA)Fe3+.
B)Fe2+.
C)Br-.
D)Al3+.
E)Al.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
36
Consider an electrochemical cell based on the following cell diagram: Pt | Pu3+(aq), Pu4+(aq)|| Cl2(g), Cl- (aq)| Pt
Given that the standard cell emf is 0.35 V and that the standard reduction potential of chlorine is 1.36 V, what is the standard reduction potential E°(Pu4+/Pu3+)?
A)2.37 V
B)1.01 V
C)-1.71 V
D)-1.01 V
E)1.71 V
Given that the standard cell emf is 0.35 V and that the standard reduction potential of chlorine is 1.36 V, what is the standard reduction potential E°(Pu4+/Pu3+)?
A)2.37 V
B)1.01 V
C)-1.71 V
D)-1.01 V
E)1.71 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the following standard reduction potentials in acid solution: 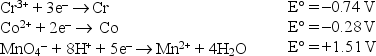 The weakest reducing agent listed above is
The weakest reducing agent listed above is
A)Cr3+.
B)Cr.
C)Mn2+.
D)Co.
E)MnO4-.
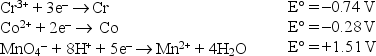 The weakest reducing agent listed above is
The weakest reducing agent listed above isA)Cr3+.
B)Cr.
C)Mn2+.
D)Co.
E)MnO4-.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
38
Consider the following electrochemical cell: U | U3+(aq)|| Cl-(aq),Cl2(g)| Pt
If the standard cell emf is 3.16 V, what is the standard reduction potential for uranium?
A)-3.16 V
B)+3.16 V
C)-1.80 V
D)+1.80 V
E)+1.36 V
If the standard cell emf is 3.16 V, what is the standard reduction potential for uranium?
A)-3.16 V
B)+3.16 V
C)-1.80 V
D)+1.80 V
E)+1.36 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
39
In the following half equation, which is the oxidizing agent? NO3-(aq)+ 4H+(aq)+ 3e- NO(g)+ 2H2O
A)NO3-
B)H+
C)e-
D)NO
E)H2O
A)NO3-
B)H+
C)e-
D)NO
E)H2O

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
40
Calculate E°cell for the following reaction: 2Fe2+(aq)+ Cd2+(aq) 2Fe3+(aq)+ Cd(s)
A)-0.37 V
B)0.37 V
C)-1.17 V
D)1.17 V
E)None of these.
A)-0.37 V
B)0.37 V
C)-1.17 V
D)1.17 V
E)None of these.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following species is the strongest oxidizing agent under standard-state conditions?
A)Ag+(aq)
B)H2(g)
C)H+(aq)
D)Cl2(g)
E)Al3+(aq)
A)Ag+(aq)
B)H2(g)
C)H+(aq)
D)Cl2(g)
E)Al3+(aq)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
42
Calculate the cell emf for the following reaction at 25°C: Ni(s)+ 2Cu2+(0.010 M) Ni2+(0.0010 M)+ 2Cu+(1.0 M)
A)0.40 V
B)-0.43 V
C)0.43 V
D)0.34 V
E)0.37 V
A)0.40 V
B)-0.43 V
C)0.43 V
D)0.34 V
E)0.37 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
43
Which one of the following reagents is capable of transforming Fe3+ (1 M)to Fe2+ (1 M)?
A)H2(1 atm)
B)NO3- (1 M)
C)O2(1 atm)
D)Br- (1 M)
E)H+ (1 M)
A)H2(1 atm)
B)NO3- (1 M)
C)O2(1 atm)
D)Br- (1 M)
E)H+ (1 M)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
44
Which one of the following reagents is capable of transforming Cu(s)to Cu2+ (1 M)?
A)I- (1 M)
B)Ni(s)
C)Ag+ (1 M)
D)Al3+ (1 M)
E)H+ (1 M)
A)I- (1 M)
B)Ni(s)
C)Ag+ (1 M)
D)Al3+ (1 M)
E)H+ (1 M)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
45
Calculate the cell emf for the following reaction: Cu2+(0.10 M)+ H2(1 atm) Cu(s)+ 2H+(pH = 3.00)
A)0.49 V
B)0.19 V
C)0.15 V
D)0.40 V
E)-0.34 V
A)0.49 V
B)0.19 V
C)0.15 V
D)0.40 V
E)-0.34 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
46
Consider the following reaction: 2Fe2+(aq)+ Cu2+ 2Fe3+(aq)+ Cu. When the reaction comes to equilibrium, what is the cell voltage?
A)0.43 V
B)1.11 V
C)0.78 V
D)-0.43 V
E)0 V
A)0.43 V
B)1.11 V
C)0.78 V
D)-0.43 V
E)0 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
47
Given the following standard reduction potentials, 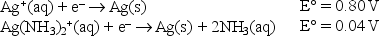 calculate the formation constant of Ag(NH3)2+ at 25°C.
calculate the formation constant of Ag(NH3)2+ at 25°C.
A)6.1 10-15
B)1.5 10-13
C)6.9 1012
D)1.6 1014
E)None of these
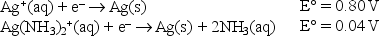 calculate the formation constant of Ag(NH3)2+ at 25°C.
calculate the formation constant of Ag(NH3)2+ at 25°C.A)6.1 10-15
B)1.5 10-13
C)6.9 1012
D)1.6 1014
E)None of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
48
For the electrochemical cell Pt(s)| H2(1 atm)| H+(1 M)|| Cu2+(1 M)| Cu(s), which one of the following changes will cause an increase in the cell voltage?
A)Lower the H2(g)pressure.
B)Increase the size/mass of the copper electrode.
C)Lower the H+(aq)concentration.
D)Decrease the concentration of Cu2+ ion.
E)None of the above.
A)Lower the H2(g)pressure.
B)Increase the size/mass of the copper electrode.
C)Lower the H+(aq)concentration.
D)Decrease the concentration of Cu2+ ion.
E)None of the above.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
49
Which one of the following reagents is capable of transforming Br- (aq)to Br2(l)under standard-state conditions?
A)I- (aq)
B)NO3- (aq)
C)Ag+ (aq)
D)Al3+ (aq)
E)Au3+ (aq)
A)I- (aq)
B)NO3- (aq)
C)Ag+ (aq)
D)Al3+ (aq)
E)Au3+ (aq)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
50
Determine the equilibrium constant, Keq, at 25°C for the reaction 2Br- (aq)+ I2(s) 
Br2(l)+ 2I- (aq)
A)5.7 10-19
B)18.30
C)1.7 1054
D)1.9 1018
E)5.7 10-55
Br2(l)+ 2I- (aq)
A)5.7 10-19
B)18.30
C)1.7 1054
D)1.9 1018
E)5.7 10-55

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
51
Using a table of standard reduction potentials, determine which of these reactions (if any)is/are nonspontaneous in the direction indicated at 25°C.
A)2Fe3+ + 2Cl- 2Fe2+ + Cl2(g)
B)2Fe3+ + 2Br- 2Fe2+ + Br2(l)
C)2Fe3+ + 2I- 2Fe2+ + I2(s)
D)A and B
E)All are spontaneous.
A)2Fe3+ + 2Cl- 2Fe2+ + Cl2(g)
B)2Fe3+ + 2Br- 2Fe2+ + Br2(l)
C)2Fe3+ + 2I- 2Fe2+ + I2(s)
D)A and B
E)All are spontaneous.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
52
Which one of the following reagents is capable of transforming Cu2+(1 M)to Cu(s)?
A)I- (1 M)
B)Ni(s)
C)Al3+ (1 M)
D)F- (1 M)
E)Ag(s)
A)I- (1 M)
B)Ni(s)
C)Al3+ (1 M)
D)F- (1 M)
E)Ag(s)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
53
Calculate the cell emf for the following reaction at 25°C: 2Ag+(0.010 M)+ H2(1 atm) 2Ag(s)+ 2H+(pH = 10.0)
A)1.04 V
B)1.27 V
C)0.92 V
D)0.56 V
E)0.80 V
A)1.04 V
B)1.27 V
C)0.92 V
D)0.56 V
E)0.80 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
54
Determine the equilibrium constant (Keq)at 25°C for the reaction Cl2(g)+ 2Br- (aq) 
2Cl- (aq)+ Br2(l)
A)1.5 10-10
B)6.3 109
C)1.3 1041
D)8.1 104
E)9.8
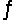
2Cl- (aq)+ Br2(l)
A)1.5 10-10
B)6.3 109
C)1.3 1041
D)8.1 104
E)9.8

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
55
For the electrochemical cell Ni(s)| Ni2+(1 M)|| H+(1 M)| H2(1 atm)| Pt(s), which one of the following changes will cause a decrease in the cell voltage?
A)Increase the pressure of H2 to 2.0 atm .
B)Decrease the mass of the nickel electrode.
C)Lower the pH of the cell electrolyte.
D)Decrease the concentration of Ni2+ ion.
E)None of the above.
A)Increase the pressure of H2 to 2.0 atm .
B)Decrease the mass of the nickel electrode.
C)Lower the pH of the cell electrolyte.
D)Decrease the concentration of Ni2+ ion.
E)None of the above.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
56
Calculate the cell voltage for the following reaction: Cu2+ (0.010 M)+ H2(1 atm) Cu(s)+ 2H+( pH = 7.0)
A)0.19 V
B)-0.01 V
C)0.34 V
D)0.69 V
E)0.49 V
A)0.19 V
B)-0.01 V
C)0.34 V
D)0.69 V
E)0.49 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
57
Consider an electrochemical cell based on the spontaneous reaction 2AgCl(s)+ Zn(s) 2Ag(s)+ 2Cl- + Zn2+.
If the zinc ion concentration is kept constant at 1 M, and the chlorine ion concentration is decreased from 1 M to 0.001 M, the cell voltage should
A)increase by 0.06 V.
B)increase by 0.18 V.
C)decrease by 0.06 V.
D)decrease by 0.18 V.
E)increase by 0.35 V.
If the zinc ion concentration is kept constant at 1 M, and the chlorine ion concentration is decreased from 1 M to 0.001 M, the cell voltage should
A)increase by 0.06 V.
B)increase by 0.18 V.
C)decrease by 0.06 V.
D)decrease by 0.18 V.
E)increase by 0.35 V.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
58
Given the following standard reduction potentials, 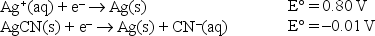 calculate the solubility product of AgCN at 25°C.
calculate the solubility product of AgCN at 25°C.
A)4.3 10-14
B)2.3 1013
C)2.1 10-14
D)5.1 1013
E)None of these
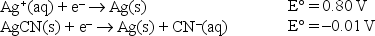 calculate the solubility product of AgCN at 25°C.
calculate the solubility product of AgCN at 25°C.A)4.3 10-14
B)2.3 1013
C)2.1 10-14
D)5.1 1013
E)None of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
59
Consider an electrochemical cell with the following cell reaction where all reactants and products are at standard-state conditions: Cu2+(aq)+ H2(g) Cu(s)+ 2H+(aq).Predict the effect on the emf of this cell of adding NaOH solution to the hydrogen half-cell until the pH equals 7.0.
A)The emf will increase.
B)The emf will decrease.
C)No change in the emf will be observed.
A)The emf will increase.
B)The emf will decrease.
C)No change in the emf will be observed.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
60
The half-cell reaction for the oxidation of H2O(l)to O2(g)is given below. 2H2O(l) O2(g)+ 4H+(aq)+ 4e-
Which choice lists all of the following species that can oxidize H2O to O2(g)under standard-state conditions?
MnO4-(aq), Cl2(g), Pb2+(aq), Cl- (aq), Ag+(aq)
A)Cl-(aq)only
B)Cl2(g)only
C)Pb2+(aq)and Ag+(aq)
D)Cl-(aq)and MnO4-(aq)
E)MnO4-(aq)and Cl2(g)
Which choice lists all of the following species that can oxidize H2O to O2(g)under standard-state conditions?
MnO4-(aq), Cl2(g), Pb2+(aq), Cl- (aq), Ag+(aq)
A)Cl-(aq)only
B)Cl2(g)only
C)Pb2+(aq)and Ag+(aq)
D)Cl-(aq)and MnO4-(aq)
E)MnO4-(aq)and Cl2(g)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
61
How many coulombs of charge are required to cause reduction of 0.20 mole of Cr3+ to Cr?
A)0.60 C
B)3.0 C
C)2.9 104 C
D)5.8 104 C
E)9.65 104 C
A)0.60 C
B)3.0 C
C)2.9 104 C
D)5.8 104 C
E)9.65 104 C

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
62
The half-reaction that occurs at the cathode during electrolysis of an aqueous CuCl2 solution is
A)Cu+ + e- Cu.
B)Cu2+ + e- Cu+.
C)2H2O + 2e- H2 + 2OH-.
D)Cl2 + 2e- 2Cl-.
E)2Cl- Cl2 + 2e-.
A)Cu+ + e- Cu.
B)Cu2+ + e- Cu+.
C)2H2O + 2e- H2 + 2OH-.
D)Cl2 + 2e- 2Cl-.
E)2Cl- Cl2 + 2e-.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
63
When an aqueous solution of AgNO3 is electrolyzed, a gas is observed to form at the anode. The gas is
A)H2.
B)O2.
C)NO.
D)NO2.
A)H2.
B)O2.
C)NO.
D)NO2.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
64
The half-reaction that should occur at the anode during electrolysis of an aqueous potassium bromide solution is
A)Br2 + 2e- 2Br-.
B)Na Na+ + e-.
C)Na+ + e- Na.
D)2Br- Br2 + 2e-.
E)2H2O O2 + 4H+ + 4e-.
A)Br2 + 2e- 2Br-.
B)Na Na+ + e-.
C)Na+ + e- Na.
D)2Br- Br2 + 2e-.
E)2H2O O2 + 4H+ + 4e-.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
65
The measured voltage of a cell in which the following reaction occurs is 0.96 V: H2(g, 1.0 atm)+ 2Ag+(aq, 1.0 M) 2H+(aq, pH = ?)+ 2Ag(s)
Calculate the pH of the H+(aq)solution.
A)1.4
B)2.7
C)5.4
D)7.1
E)14.9
Calculate the pH of the H+(aq)solution.
A)1.4
B)2.7
C)5.4
D)7.1
E)14.9

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
66
A current of 0.80 A was applied to an electrolytic cell containing molten CdCl2 for 2.5 hours.Calculate the mass of cadmium metal deposited.
A)3.2 10-7 g
B)1.2 10-3 g
C)4.2 g
D)8.4 g
E)16.8 g
A)3.2 10-7 g
B)1.2 10-3 g
C)4.2 g
D)8.4 g
E)16.8 g

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
67
Consider an electrochemical cell involving the overall reaction 2AgBr(s)+ Pb(s) Pb2+ + 2Ag(s)+ 2Br-
Each half-reaction is carried out in a separate compartment. The anion included in the lead half-cell is NO3-. The cation in the silver half-cell is K+. The two half-cells are connected by a KNO3 salt bridge. If [Pb2+] = 1.0 M, what concentration of Br- ion will produce a cell emf of 0.25 V at 298 K?
Given: AgBr(s)+ e- Ag + Br-, E° = +0.07 V.
A)0.02 M
B)0.14 M
C)0.38 M
D)1.0 M
E)7.0 M
Each half-reaction is carried out in a separate compartment. The anion included in the lead half-cell is NO3-. The cation in the silver half-cell is K+. The two half-cells are connected by a KNO3 salt bridge. If [Pb2+] = 1.0 M, what concentration of Br- ion will produce a cell emf of 0.25 V at 298 K?
Given: AgBr(s)+ e- Ag + Br-, E° = +0.07 V.
A)0.02 M
B)0.14 M
C)0.38 M
D)1.0 M
E)7.0 M

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
68
How many coulombs of charge are required to cause reduction of 0.25 mole of Cu2+ to Cu?
A)0.25 C
B)0.50 C
C)1.2 104 C
D)2.4 104 C
E)4.8 104 C
A)0.25 C
B)0.50 C
C)1.2 104 C
D)2.4 104 C
E)4.8 104 C

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
69
A metal object is to be gold-plated by an electrolytic procedure using aqueous AuCl3 electrolyte. Calculate the number of moles of gold deposited in 3.0 min by a constant current of 10.A.
A)6.2 10-3 mol
B)9.3 10-3 mol
C)1.8 10-2 mol
D)3.5 10-5 mol
E)160 mol
A)6.2 10-3 mol
B)9.3 10-3 mol
C)1.8 10-2 mol
D)3.5 10-5 mol
E)160 mol

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
70
The half-reaction occurring at the cathode during electrolysis of an aqueous copper(II)iodide solution is
A)I2 + 2e- 2I-.
B)Cu Cu2+ + 2e-.
C)Cu2+ + 2e- Cu.
D)2I- I2 + 2e-.
E)2e- + 2H2O H2 + 2OH-.
A)I2 + 2e- 2I-.
B)Cu Cu2+ + 2e-.
C)Cu2+ + 2e- Cu.
D)2I- I2 + 2e-.
E)2e- + 2H2O H2 + 2OH-.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
71
How many faradays are transferred in an electrolytic cell when a current of 2.0 amperes flows for 12 hours?
A)24 F
B)8.6 104 F
C)0.90 F
D)6.2 10 -3 F
E)1.1 F
A)24 F
B)8.6 104 F
C)0.90 F
D)6.2 10 -3 F
E)1.1 F

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
72
A current of 2.50 A was passed through an electrolytic cell containing molten CaCl2 for 4.50 hours. How many moles of calcium metal should be deposited?
A)5.83 10-5 mol
B)0.210 mol
C)0.420 mol
D)0.840 mol
E)1.95 109 mol
A)5.83 10-5 mol
B)0.210 mol
C)0.420 mol
D)0.840 mol
E)1.95 109 mol

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
73
Calculate the minimum voltage required for the electrolysis of 1.0 M NaCl in neutral solution. 2H2O + 2Cl- (1.0 M) H2(1 atm)+ Cl2(1 atm)+ 2OH- (1 10-7 M)
A)2.19 V
B)1.78 V
C)0.41 V
D)-0.41 V
E)-1.78 V
A)2.19 V
B)1.78 V
C)0.41 V
D)-0.41 V
E)-1.78 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
74
Under standard-state conditions, which of the following half-reactions occurs at the cathode during the electrolysis of aqueous nickel sulfate at 25°C?
A)2H2O O2 + 4H+ + 4e-
B)Ni2+ + 2e- Ni
C)2H2O + 2e- H2 + 2OH-
D)Ni Ni2+ + 2e-
A)2H2O O2 + 4H+ + 4e-
B)Ni2+ + 2e- Ni
C)2H2O + 2e- H2 + 2OH-
D)Ni Ni2+ + 2e-

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
75
The half-reaction that occurs at the cathode during electrolysis of an aqueous sodium iodide solution is
A)Na+ + e- Na.
B)Na Na+ + e-.
C)2H2O + 2e- H2 + 2OH-.
D)I2 + 2e- 2I-.
E)2I- I2 + 2e-.
A)Na+ + e- Na.
B)Na Na+ + e-.
C)2H2O + 2e- H2 + 2OH-.
D)I2 + 2e- 2I-.
E)2I- I2 + 2e-.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
76
Which one of the following reactions must be carried out in an electrolytic cell rather than in a galvanic cell?
A)Zn2+ + Ca Zn + Ca2+
B)Al3+ + 3Br- Al + (3/2)Br2
C)2Al + 3Fe2+ 2Al3+ + 3Fe
D)H2 + I2(s) 2H+ + 2I-
A)Zn2+ + Ca Zn + Ca2+
B)Al3+ + 3Br- Al + (3/2)Br2
C)2Al + 3Fe2+ 2Al3+ + 3Fe
D)H2 + I2(s) 2H+ + 2I-

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
77
The measured voltage of the cell Pt(s)| H2 (1.0 atm)| H+(aq)|| Ag+(1.0 M)| Ag(s)is 1.02 V at 25°C. Calculate the pH of the solution.
A)1.86
B)1.69
C)3.72
D)3.89
E)7.43
A)1.86
B)1.69
C)3.72
D)3.89
E)7.43

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
78
Predict the products obtained from electrolysis of a 1 M AlBr3 solution. Note that 2H2O(l)+ 2e- H2(g)+ 2OH-(aq), E°red = -0.83 V, and
O2(g)+ 4H+(aq)+ 4e- 2H2O(l), E°red = +1.23 V
A)Al and Br2
B)Al and O2
C)H2 and O2
D)H2 and Br2
E)Al and H2
O2(g)+ 4H+(aq)+ 4e- 2H2O(l), E°red = +1.23 V
A)Al and Br2
B)Al and O2
C)H2 and O2
D)H2 and Br2
E)Al and H2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
79
If the measured voltage of the cell Zn(s)| Zn2+(aq)|| Ag+(aq)| Ag(s)is 1.37 V when the concentration of Zn2+ ion is 0.010 M, what is the Ag+ ion concentration?
A)2.5 M
B)4.0 10-9 M
C)6.2 10-3 M
D)2.6 10-51 M
E)6.2 10-5 M
A)2.5 M
B)4.0 10-9 M
C)6.2 10-3 M
D)2.6 10-51 M
E)6.2 10-5 M

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
80
Predict the products of the electrolysis of aqueous aluminum bromide AlBr3(aq). (Balancing is not required.)
A)Al + Br2
B)Al + O2 + H+
C)H2 + OH- + Br2
D)H2 + O2
A)Al + Br2
B)Al + O2 + H+
C)H2 + OH- + Br2
D)H2 + O2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck



