Deck 24: Organic Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/57
Play
Full screen (f)
Deck 24: Organic Chemistry
1
The two molecules represented below are examples of CH3-CH2-O-CH2CH3 CH3CH2CH2CH2-OH
A)geometric isomers.
B)structural isomers.
C)optical isomers.
D)stereoisomers.
E)None of these.
A)geometric isomers.
B)structural isomers.
C)optical isomers.
D)stereoisomers.
E)None of these.
structural isomers.
2
Unsaturated hydrocarbons
A)contain at least one double or triple carbon-carbon bond.
B)contain at least one element other than hydrogen and carbon.
C)contain the maximum number of hydrogens that can bond with the carbon atoms present.
D)cannot form structural isomers.
E)cannot undergo addition reactions.
A)contain at least one double or triple carbon-carbon bond.
B)contain at least one element other than hydrogen and carbon.
C)contain the maximum number of hydrogens that can bond with the carbon atoms present.
D)cannot form structural isomers.
E)cannot undergo addition reactions.
contain at least one double or triple carbon-carbon bond.
3
The octane rating of gasoline refers to its
A)percentage C8H18 by volume.
B)radiation dose.
C)alcohol level.
D)ability to resist engine knocking.
E)percentage of unsaturated hydrocarbons.
A)percentage C8H18 by volume.
B)radiation dose.
C)alcohol level.
D)ability to resist engine knocking.
E)percentage of unsaturated hydrocarbons.
ability to resist engine knocking.
4
How many structural isomers are there of C4H10?
A)4
B)6
C)2
D)8
E)10
A)4
B)6
C)2
D)8
E)10

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
5
Which of these molecules is unsaturated?
A)CH4
B)C2H6
C)C4H6
D)C5H12
E)C6H14
A)CH4
B)C2H6
C)C4H6
D)C5H12
E)C6H14

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
6
Cycloalkanes have the general formula
A)CnH2n-4
B)CnH2n-2
C)CnH2n
D)CnH2n+2
E)CnH2n+4
A)CnH2n-4
B)CnH2n-2
C)CnH2n
D)CnH2n+2
E)CnH2n+4

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
7
The compound that has a triple bond between one pair of carbon atoms is called a/an
A)alkane.
B)chlorofluorocarbon.
C)alkyne.
D)alkene.
E)alcohol.
A)alkane.
B)chlorofluorocarbon.
C)alkyne.
D)alkene.
E)alcohol.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
8
Which of these pairs are geometric isomers?
A)CH3CH2-O-CH2CH3 and CH3CH2CH2CH2OH
B)
C)
D)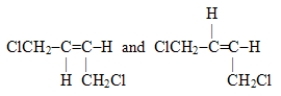
A)CH3CH2-O-CH2CH3 and CH3CH2CH2CH2OH
B)

C)

D)


Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
9
The formula CH3CH2CH2CH=CH2 represents
A)an alkane.
B)a cycloalkane.
C)an alkene.
D)an alkyne.
E)an aromatic compound.
A)an alkane.
B)a cycloalkane.
C)an alkene.
D)an alkyne.
E)an aromatic compound.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
10
A particular structural isomer of C6H14 is shown below. 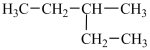 Which of the following structures represents a different structural isomer of C6H14 than the one shown above?
Which of the following structures represents a different structural isomer of C6H14 than the one shown above? 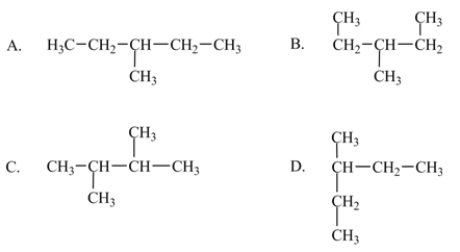
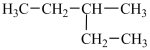 Which of the following structures represents a different structural isomer of C6H14 than the one shown above?
Which of the following structures represents a different structural isomer of C6H14 than the one shown above? 

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
11
The alkane with six carbon atoms is called
A)butane.
B)hexane.
C)heptane.
D)butene.
E)none of these.
A)butane.
B)hexane.
C)heptane.
D)butene.
E)none of these.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
12
Which of these species is an aromatic compound?
A)C2H2
B)C6H12
C)C6H4Br2
D)C5H10
E)C2H4Br2
A)C2H2
B)C6H12
C)C6H4Br2
D)C5H10
E)C2H4Br2

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
13
Alkynes have the general formula
A)CnH2n-4
B)CnH2n-2
C)CnH2n
D)CnH2n+2
E)CnH2n+4
A)CnH2n-4
B)CnH2n-2
C)CnH2n
D)CnH2n+2
E)CnH2n+4

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
14
The systematic name for the compound represented below is 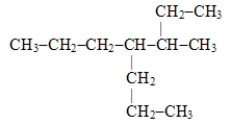
A)4,5-diethylheptane
B)3-propyl-4-ethylhexane
C)3-ethyl-4-propylhexane
D)3-methyl-4-propylheptane
E)2-ethyl-4-propylhexane

A)4,5-diethylheptane
B)3-propyl-4-ethylhexane
C)3-ethyl-4-propylhexane
D)3-methyl-4-propylheptane
E)2-ethyl-4-propylhexane

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
15
Which one of these hydrocarbon chains would have the highest octane rating?
A)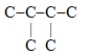
B)C-C-C-C-C-C
C)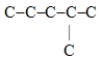
D)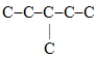
A)
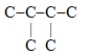
B)C-C-C-C-C-C
C)
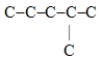
D)
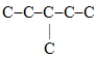

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
16
Which of these is the systematic name for the compound represented below? 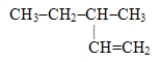
A)2-ethylbutane
B)3-methylpentene
C)3-methyl-1-pentene
D)3-methyl-1-hexene
E)2-methylhexane
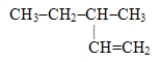
A)2-ethylbutane
B)3-methylpentene
C)3-methyl-1-pentene
D)3-methyl-1-hexene
E)2-methylhexane

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
17
Alkanes have the general formula
A)CnH2n-4
B)CnH2n-2
C)CnH2n
D)CnH2n+2
E)CnH2n+4
A)CnH2n-4
B)CnH2n-2
C)CnH2n
D)CnH2n+2
E)CnH2n+4

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
18
Alkenes have the general formula
A)CnH2n-4
B)CnH2n-2
C)CnH2n
D)CnH2n+2
E)CnH2n+4
A)CnH2n-4
B)CnH2n-2
C)CnH2n
D)CnH2n+2
E)CnH2n+4

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
19
The two molecules represented below are examples of 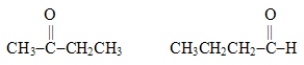
A)isomers
B)isotopes
C)alcohols
D)carboxylic acids
E)unsaturated hydrocarbons
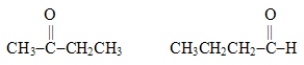
A)isomers
B)isotopes
C)alcohols
D)carboxylic acids
E)unsaturated hydrocarbons

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
20
Which one of these hydrocarbons does not have isomers?
A)C7H16
B)C6H14
C)C5H10
D)C4H8
E)C3H8
A)C7H16
B)C6H14
C)C5H10
D)C4H8
E)C3H8

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
21
Which is the product of the reaction of one mole of HCl with one mole of 1-butyne?
A)1-chloro-1-butene
B)1-chloro-2-butene
C)2-chloro-1-butene
D)ethyl chloride + acetylene
A)1-chloro-1-butene
B)1-chloro-2-butene
C)2-chloro-1-butene
D)ethyl chloride + acetylene

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
22
"Wood alcohol" is the common name for
A)methanol.
B)ethanol.
C)propyl alcohol.
D)ethylene.
E)acetylene.
A)methanol.
B)ethanol.
C)propyl alcohol.
D)ethylene.
E)acetylene.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
23
Which one of the following functional groups is found in carboxylic acids? 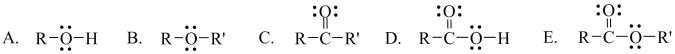
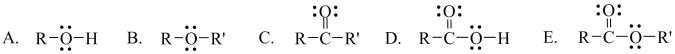

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
24
The correct structure for 2,3,3-trimethylpentane is
A)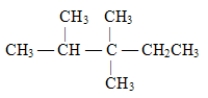
B)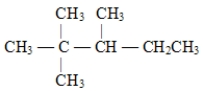
C)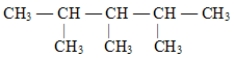
D)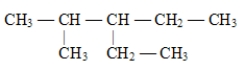
A)
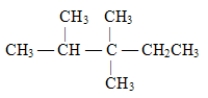
B)
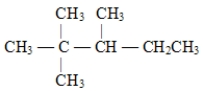
C)

D)
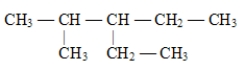

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
25
The reaction of ethylene and water yields
A)an aldehyde.
B)an ester.
C)an alcohol.
D)an ether.
E)an organic acid.
A)an aldehyde.
B)an ester.
C)an alcohol.
D)an ether.
E)an organic acid.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
26
Which one of these structures represents an ester functional group? 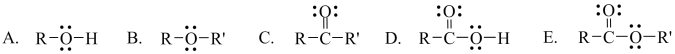
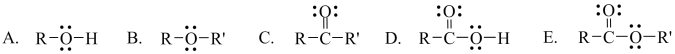

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
27
The reaction of an alcohol and a carboxylic acid yields
A)a hydrocarbon.
B)an ester.
C)an ether.
D)an aldehyde.
E)a ketone.
A)a hydrocarbon.
B)an ester.
C)an ether.
D)an aldehyde.
E)a ketone.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
28
The group of atoms that is responsible for the characteristic properties of a family of organic compounds is called a/an
A)reaction center.
B)functional group.
C)binding site.
D)enzyme.
E)polyatomic ion.
A)reaction center.
B)functional group.
C)binding site.
D)enzyme.
E)polyatomic ion.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
29
Oxidation of the 2-propanol will produce a/an
A)aldehyde.
B)amine.
C)alkene.
D)ketone.
E)carboxylic acid.
A)aldehyde.
B)amine.
C)alkene.
D)ketone.
E)carboxylic acid.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
30
Which one of the following functional groups is found in alcohols. 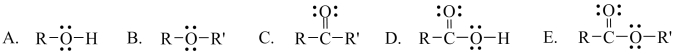
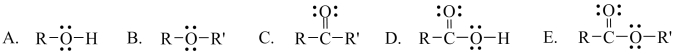

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
31
Which of these is the systematic name for the compound represented below? 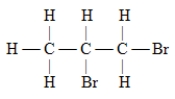
A)2,3-dibromopentane
B)1,2-dibromopentane
C)2,3-dibromopropane
D)1,2-propane dibromide
E)1,2-dibromopropane
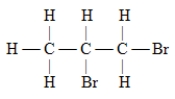
A)2,3-dibromopentane
B)1,2-dibromopentane
C)2,3-dibromopropane
D)1,2-propane dibromide
E)1,2-dibromopropane

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
32
Which one of these choices is the formula for an aldehyde?
A)CH3CHO
B)CH3OCH3
C)CH3COCH3
D)CH3COOH
E)HC CH
A)CH3CHO
B)CH3OCH3
C)CH3COCH3
D)CH3COOH
E)HC CH

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
33
Acetylene, C2H2, the simplest alkyne, can be prepared from "inorganic" materials. Which of these reactions is used to prepare acetylene in this way?
A)2C + H2 C2H2
B)C2H4 C2H2 + H2
C)2CO + 2H2O C2H2 + H2O2
D)CaC2 + 2H2O C2H2 + Ca(OH)2
A)2C + H2 C2H2
B)C2H4 C2H2 + H2
C)2CO + 2H2O C2H2 + H2O2
D)CaC2 + 2H2O C2H2 + Ca(OH)2

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
34
The expected product from the addition of HCl to CH3-CH2-CH=CH2 is
A)CH3-CH2-CH=CHCl
B)CH3-CH2-CCl=CH2
C)CH3-CHCl-CH=CH2
D)CH3-CH2-CH2-CH2Cl
E)CH3-CH2-CHCl-CH3
A)CH3-CH2-CH=CHCl
B)CH3-CH2-CCl=CH2
C)CH3-CHCl-CH=CH2
D)CH3-CH2-CH2-CH2Cl
E)CH3-CH2-CHCl-CH3

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
35
Esters are synthesized from two classes of organic compounds. Those two types of compounds are
A)acids and bases.
B)amines and alcohols.
C)alcohols and acids.
D)amines and alkenes.
E)alkenes and bases.
A)acids and bases.
B)amines and alcohols.
C)alcohols and acids.
D)amines and alkenes.
E)alkenes and bases.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
36
Which type of organic compound does not contain a carbonyl group?
A)ethers
B)carboxylic acids
C)ketones
D)aldehydes
E)esters
A)ethers
B)carboxylic acids
C)ketones
D)aldehydes
E)esters

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
37
The name for the compound with the formula CH3CH2CH2CH2OH is
A)propanol.
B)propane.
C)butanol.
D)pentane.
E)pentanol.
A)propanol.
B)propane.
C)butanol.
D)pentane.
E)pentanol.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
38
Which one of the following functional groups is found in ketones? 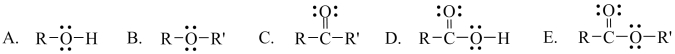
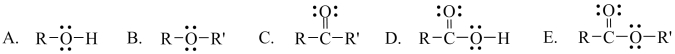

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
39
The reaction of Cl2 with CH4 to produce methyl chloride is an example of a/an
A)free radical reaction.
B)addition reaction.
C)reduction reaction.
D)ester hydrolysis.
E)polymerization.
A)free radical reaction.
B)addition reaction.
C)reduction reaction.
D)ester hydrolysis.
E)polymerization.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
40
Which choice gives the structures of the reaction products when the ester below is hydrolyzed in acid solution? 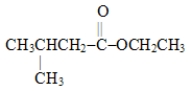
A)
B)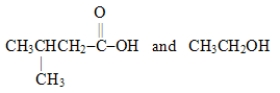
C)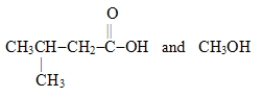
D)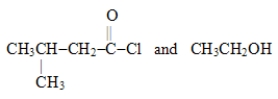
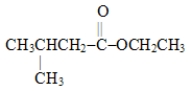
A)

B)
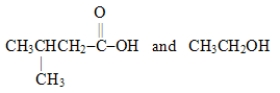
C)
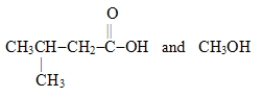
D)
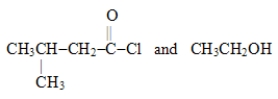

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
41
The oxidation product of 1-propanol when using Cr2O72- as the oxidizing agent is acetone.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
42
The systematic name for the compound with the following structural formula is 4,5-dimethyl-2-hexene. 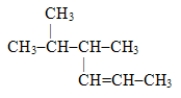
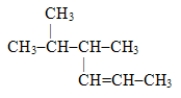

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
43
The reaction of hydrogen chloride gas with propene will yield 1-chloropropane as the main product.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
44
Name the following compound: 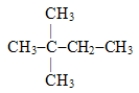
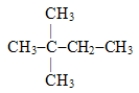

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
45
Which functional group, when present in a compound that is allowed to stand in air, poses a danger of slowly yielding explosive peroxides?
A)ether
B)alcohol
C)carboxylic acid
D)ketone
E)unsaturated hydrocarbon
A)ether
B)alcohol
C)carboxylic acid
D)ketone
E)unsaturated hydrocarbon

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
46
Name the following compound: 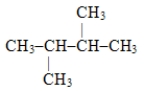


Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
47
A compound with the formula C6H12 may or may not be a saturated hydrocarbon.Explain.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
48
Which of these reactions leads to a change in the hybridization of one or more carbon atoms?
A)free radical halogenation of an alkane
B)hydrolysis of an ester to yield an acid and an alcohol
C)substitution of an aromatic ring using a halogen
D)oxidation of an alcohol to yield a carboxylic acid
E)neutralization of an amine using a strong mineral acid
A)free radical halogenation of an alkane
B)hydrolysis of an ester to yield an acid and an alcohol
C)substitution of an aromatic ring using a halogen
D)oxidation of an alcohol to yield a carboxylic acid
E)neutralization of an amine using a strong mineral acid

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
49
Write the formula for the alcohol and the carboxylic acid from which the following ester may be synthesized. 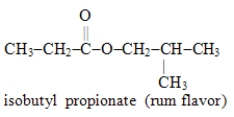


Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
50
The molecule shown below is chiral, i.e.not superimposable on its mirror image. 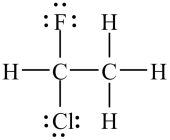


Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
51
The systematic name for the hydrocarbon with the following structural formula is 1-ethyl-2-methylbutane. 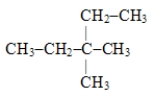


Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
52
Bromination of benzene (C6H6), an aromatic compound,
A)occurs by substitution rather than addition.
B)occurs by addition rather than substitution.
C)occurs more rapidly than bromination of a nonaromatic compound.
D)results in formation of 1,2,3,4,5,6-hexabromocyclohexane.
E)occurs in the absence of a catalyst.
A)occurs by substitution rather than addition.
B)occurs by addition rather than substitution.
C)occurs more rapidly than bromination of a nonaromatic compound.
D)results in formation of 1,2,3,4,5,6-hexabromocyclohexane.
E)occurs in the absence of a catalyst.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
53
Write the formula for the alcohol and the carboxylic acid from which the following ester may be synthesized. 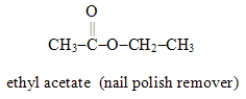
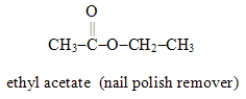

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following compounds are isomers of each other?
I.pentane
II.2-methylbutane
III.2,3-dimethylbutane
IV.2,2-dimethylpropane
V.1-hexene
I.pentane
II.2-methylbutane
III.2,3-dimethylbutane
IV.2,2-dimethylpropane
V.1-hexene

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
55
Amines are
A)organic bases that react with water to produce ammonia.
B)organic acids that react with water to produce ammonia.
C)organic bases that react with acids to form ammonium salts.
D)organic acids that react with bases to form ammonium salts.
E)None of the above.
A)organic bases that react with water to produce ammonia.
B)organic acids that react with water to produce ammonia.
C)organic bases that react with acids to form ammonium salts.
D)organic acids that react with bases to form ammonium salts.
E)None of the above.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
56
Write the formula for the alcohol and the carboxylic acid from which the following ester may be synthesized. 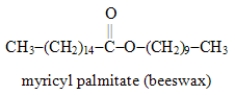
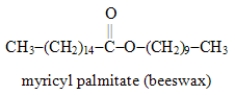

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
57
Which of these statements describes a condensation reaction?
A)addition of H2O to a double bond
B)linking an acid and an alcohol to make an ester and water
C)addition of H2 to an alkene
D)oxidation of ethanol to acetaldehyde
E)hydrolysis of an ester
A)addition of H2O to a double bond
B)linking an acid and an alcohol to make an ester and water
C)addition of H2 to an alkene
D)oxidation of ethanol to acetaldehyde
E)hydrolysis of an ester

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck



