Deck 12: Organic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/162
Play
Full screen (f)
Deck 12: Organic Compounds
1
What would the formula be for the following stick model? 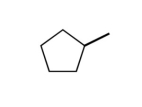
A)C6H12
B)C5H5
C)C6H10
D)C5H10
E)none of the above

A)C6H12
B)C5H5
C)C6H10
D)C5H10
E)none of the above
C6H12
2
Which of the following molecules is a branched hydrocarbon? 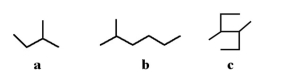
A)a
B)b
C)c
D)All of the above are branched hydrocarbons.
E)only a and b

A)a
B)b
C)c
D)All of the above are branched hydrocarbons.
E)only a and b
All of the above are branched hydrocarbons.
3
Which of the following statements describes straight-chain hydrocarbon?
A)a hydrocarbon where every carbon has only a single bond to no more than two other carbons
B)a hydrocarbon where every carbon is bound with a straight line to every other carbon
C)a carbon atom that is bound straight to four hydrogens
D)a hydrocarbon that has a linear rod shaped conformation
E)none of the above
A)a hydrocarbon where every carbon has only a single bond to no more than two other carbons
B)a hydrocarbon where every carbon is bound with a straight line to every other carbon
C)a carbon atom that is bound straight to four hydrogens
D)a hydrocarbon that has a linear rod shaped conformation
E)none of the above
a hydrocarbon where every carbon has only a single bond to no more than two other carbons
4
Which of the following molecules could be a different conformation of the underlined molecule below? 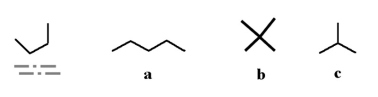
A)a
B)b
C)c
D)all of the above
E)none of the above

A)a
B)b
C)c
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following two stick structures are different conformations of the same molecule? 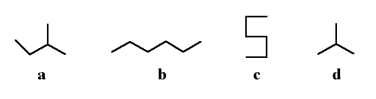
A)a and c
B)b and c
C)a and d
D)b and d
E)none of the above

A)a and c
B)b and c
C)a and d
D)b and d
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
6
A change in configuration, but not a change in conformation, involves ________.
A)a change in spatial orientation
B)the breaking of a bond
C)both the breaking and forming of a bond
D)a change in chemical formula
A)a change in spatial orientation
B)the breaking of a bond
C)both the breaking and forming of a bond
D)a change in chemical formula

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following two stick structures are structural isomers? 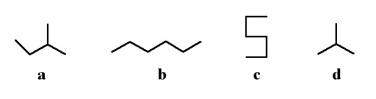
A)a and b
B)a and c
C)b and c
D)b and d
E)none of the above

A)a and b
B)a and c
C)b and c
D)b and d
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements describes structural isomers?
A)two or more compounds with the same number and type of atoms but different connectivity
B)two or more compounds with the same shape
C)two compounds with the same number of conformations
D)two or more compounds with different atoms but with the same electronic properties
E)all of the above
A)two or more compounds with the same number and type of atoms but different connectivity
B)two or more compounds with the same shape
C)two compounds with the same number of conformations
D)two or more compounds with different atoms but with the same electronic properties
E)all of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is the simplest possible hydrocarbon?
A)CH4
B)H2
C)HC CH
CH
D)H2C CH2
CH2
E)C2H6
A)CH4
B)H2
C)HC
 CH
CHD)H2C
 CH2
CH2E)C2H6

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements describes branched hydrocarbons?
A)a hydrocarbon where carbon has a single bond to more than two other carbons
B)a hydrocarbon where every carbon is bound with a crooked line to every other carbon
C)a carbon atom that is branched
D)a hydrocarbon that has a branched shaped conformation
E)a hydrocarbon that is bound to Br
A)a hydrocarbon where carbon has a single bond to more than two other carbons
B)a hydrocarbon where every carbon is bound with a crooked line to every other carbon
C)a carbon atom that is branched
D)a hydrocarbon that has a branched shaped conformation
E)a hydrocarbon that is bound to Br

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements best describes the concept of structural conformation?
A)rearranging the shape of a molecule by rotating around bonds
B)rearranging the shape of a molecule by changing the arrangement of bonds
C)changing the structure by adding or subtracting bonds
D)arranging the atom into the best conformation for the given structure
E)changing the conformation to generate a new molecule with different chemical properties
A)rearranging the shape of a molecule by rotating around bonds
B)rearranging the shape of a molecule by changing the arrangement of bonds
C)changing the structure by adding or subtracting bonds
D)arranging the atom into the best conformation for the given structure
E)changing the conformation to generate a new molecule with different chemical properties

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
12
What would the formula be for the following stick model? 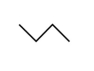
A)C4H10
B)C3H3
C)C3H6
D)C3H8
E)C4H8

A)C4H10
B)C3H3
C)C3H6
D)C3H8
E)C4H8

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
13
What is a hydrocarbon?
A)It is a molecule composed of carbon and hydrogen only.
B)It is a wet carbon atom
C)It is any organic molecule.
D)It is a molecule derived from hydrogen synthesis.
E)none of the above
A)It is a molecule composed of carbon and hydrogen only.
B)It is a wet carbon atom
C)It is any organic molecule.
D)It is a molecule derived from hydrogen synthesis.
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following molecules could be a different conformation of the underlined molecule below? 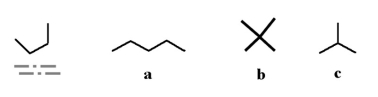
A)a
B)b
C)c
D)all of the above
E)none of the above

A)a
B)b
C)c
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following molecules could be a structural isomer for the underlined molecule below? 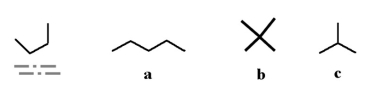
A)a
B)b
C)c
D)all of the above
E)none of the above

A)a
B)b
C)c
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following stick structures could describe pentane (C5H12)? 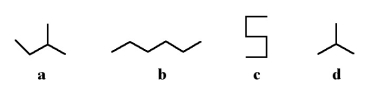
A)a
B)b
C)c
D)d
E)none of the above

A)a
B)b
C)c
D)d
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
17
What determines the chemical and physical properties of hydrocarbons?
A)the way the atoms are connected together
B)the number of carbon and hydrogens
C)the elements it is composed of
D)the number of oxygen
E)both A and B
A)the way the atoms are connected together
B)the number of carbon and hydrogens
C)the elements it is composed of
D)the number of oxygen
E)both A and B

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following molecules is not a straight-chain hydrocarbon? 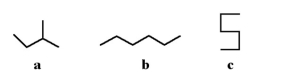
A)a
B)b
C)c
D)All of the above are straight-chain hydrocarbons.
E)None of the above are straight-chain hydrocarbons.

A)a
B)b
C)c
D)All of the above are straight-chain hydrocarbons.
E)None of the above are straight-chain hydrocarbons.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
19
An organic compound is any molecule ________.
A)with a structure based upon carbon
B)grown with organic farming methods
C)isolated from living organisms, including water
D)consisting primarily of orgonium atoms
E)that is a salt obtained from organic creatures
A)with a structure based upon carbon
B)grown with organic farming methods
C)isolated from living organisms, including water
D)consisting primarily of orgonium atoms
E)that is a salt obtained from organic creatures

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following molecules could be a structural isomer for C5H12? 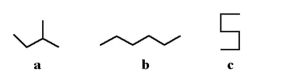
A)a
B)b
C)c
D)all of the above
E)none of the above

A)a
B)b
C)c
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
21
How many structural isomers are there for hydrocarbons having the molecular formula C6H14?
A)three
B)four
C)five
D)six
A)three
B)four
C)five
D)six

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
22
Which two of these four structures are of the same structural isomer? 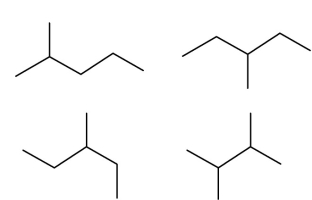
A)upper right and lower left
B)upper left and lower right
C)lower left and upper right
D)lower right and upper right

A)upper right and lower left
B)upper left and lower right
C)lower left and upper right
D)lower right and upper right

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
23
Where would you expect to isolate molecules in a petroleum fractionating tower that have the strongest intermolecular forces?
A)near the bottom
B)near the top
C)near the middle
D)Fractional distillation takes advantage of molecular weight.
E)Fractional distillation takes advantage of the number of isomers.
A)near the bottom
B)near the top
C)near the middle
D)Fractional distillation takes advantage of molecular weight.
E)Fractional distillation takes advantage of the number of isomers.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
24
How are most hydrocarbons made?
A)They are isolated from fossil fuels.
B)They are made by plants.
C)They are made at refineries.
D)They are made in swamps.
E)none of the above
A)They are isolated from fossil fuels.
B)They are made by plants.
C)They are made at refineries.
D)They are made in swamps.
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
25
How many structural isomers are there for hydrocarbons having the molecular formula C4H10?
A)none
B)one
C)two
D)three
A)none
B)one
C)two
D)three

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
26
In a fractionating tower, the crude oil vapors pass from a pipe still into the column. Tar and lubricating stock are the first components to be pulled off at the bottom. Nearer the top kerosene is pulled off followed by gasoline and finally natural gas at the very top. From this information, which has a higher boiling point, gasoline or kerosine?
A)Gasoline has the higher boiling point.
B)Kerosene has the higher boiling point.
C)Their boiling points are the same, but kerosene has the greater density.
D)Fractional distillation components are pulled off based on molecular weight, so it is not possible to know which has the higher boiling point from the information given.
A)Gasoline has the higher boiling point.
B)Kerosene has the higher boiling point.
C)Their boiling points are the same, but kerosene has the greater density.
D)Fractional distillation components are pulled off based on molecular weight, so it is not possible to know which has the higher boiling point from the information given.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following molecules would probably be isolated closest to the top of a fractionating tower at a refinery?
A)C4H10
B)C8H18
C)C10H22
D)C20H42
E)C40H82
A)C4H10
B)C8H18
C)C10H22
D)C20H42
E)C40H82

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
28
How many structural isomers are shown here? 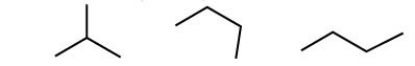
A)one
B)two
C)three
D)four

A)one
B)two
C)three
D)four

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the above stick structures would have the highest octane rating for use in your engine?
A)a
B)b
C)c
D)All of the above have the same octane rating.
E)None of the above are octane.
A)a
B)b
C)c
D)All of the above have the same octane rating.
E)None of the above are octane.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
30
Why does the melting point of hydrocarbons increase as the number of carbon atoms per molecule increases?
A)An increase in the number of carbon atoms per molecules also means an increase in the density of the hydrocarbon.
B)because of greater induced dipole-induced dipole molecular attractions
C)Larger hydrocarbon chains tend to be branched.
D)The molecular mass also increases.
A)An increase in the number of carbon atoms per molecules also means an increase in the density of the hydrocarbon.
B)because of greater induced dipole-induced dipole molecular attractions
C)Larger hydrocarbon chains tend to be branched.
D)The molecular mass also increases.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
31
What is the chemical cause of engine knock?
A)the straight chain isomers igniting too early in the engine
B)the branched isomers igniting too early in the engine
C)the wrong conformer igniting too early in the engine
D)too much oxygen in your piston
E)the octane igniting too early in your engine
A)the straight chain isomers igniting too early in the engine
B)the branched isomers igniting too early in the engine
C)the wrong conformer igniting too early in the engine
D)too much oxygen in your piston
E)the octane igniting too early in your engine

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
32
What property of carbon allows for the formation of so many different organic molecules?
A)Carbon forms three different isotopes which allows for the formation of many more molecules than other elements.
B)Carbon atoms are unique in their ability to form strong chemical bonds repeatedly with other carbon atoms, which permits the formation of countless possible structures.
C)Carbon atoms uniquely contain exactly the same number (six)of protons, neutrons and electrons in their atomic structure.
D)Carbon resides in the exact middle of the periodic table, from left to right, with exactly the same number of reactive elements on either side of it. It can therefore react with the maximum number of elements.
A)Carbon forms three different isotopes which allows for the formation of many more molecules than other elements.
B)Carbon atoms are unique in their ability to form strong chemical bonds repeatedly with other carbon atoms, which permits the formation of countless possible structures.
C)Carbon atoms uniquely contain exactly the same number (six)of protons, neutrons and electrons in their atomic structure.
D)Carbon resides in the exact middle of the periodic table, from left to right, with exactly the same number of reactive elements on either side of it. It can therefore react with the maximum number of elements.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
33
How many compounds have the chemical formula 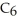
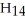 ?
?
A)2
B)3
C)5
D)8
E)12
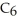
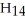 ?
?A)2
B)3
C)5
D)8
E)12

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the above stick structures would have the lowest octane rating for use in your engine?
A)a
B)b
C)c
D)All of the above have the same octane rating.
E)None of the above are octane.
A)a
B)b
C)c
D)All of the above have the same octane rating.
E)None of the above are octane.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
35
Which bond would you rotate around to generate the structure on the right? 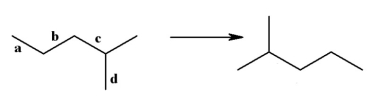
A)a
B)b
C)c
D)d
E)No specific bond. Just flip the entire structure.

A)a
B)b
C)c
D)d
E)No specific bond. Just flip the entire structure.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
36
Which bond would you rotate around to generate the structure on the right? 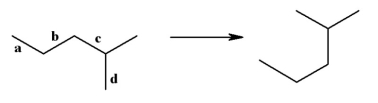
A)a
B)b
C)c
D)d
E)none of the above

A)a
B)b
C)c
D)d
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
37
What is meant by the term octane rating?
A)the degree of branching in the carbon framework
B)the degree at which the compound ignites
C)the percentage of octane in the mixture
D)a measure of how many degrees it takes to ignite a mixture of octane
E)none of the above
A)the degree of branching in the carbon framework
B)the degree at which the compound ignites
C)the percentage of octane in the mixture
D)a measure of how many degrees it takes to ignite a mixture of octane
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following molecules would probably be isolated closest to the bottom of a fractionating tower at a refinery?
A)C4H10
B)C8H18
C)C10H22
D)C20H42
E)C40H82
A)C4H10
B)C8H18
C)C10H22
D)C20H42
E)C40H82

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
39
Where would you expect to isolate molecules in a fractionating tower that have very few interatomic attractions?
A)near the bottom
B)near the top
C)near the middle
D)Fractional distillation takes advantage of molecular weight.
E)Fractional distillation takes advantage of the number of isomers.
A)near the bottom
B)near the top
C)near the middle
D)Fractional distillation takes advantage of molecular weight.
E)Fractional distillation takes advantage of the number of isomers.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
40
How does fractional distillation work?
A)It takes advantage of the different boiling points of molecules to separate them.
B)It takes advantage of the different weight of molecules to separate them.
C)It uses the fraction of carbon in the isomers to separate the molecules.
D)It takes advantage of the different melting points to separate molecules.
E)none of the above
A)It takes advantage of the different boiling points of molecules to separate them.
B)It takes advantage of the different weight of molecules to separate them.
C)It uses the fraction of carbon in the isomers to separate the molecules.
D)It takes advantage of the different melting points to separate molecules.
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the above structures should be named as a hexane?
A)a
B)b
C)c
D)None of the above
A)a
B)b
C)c
D)None of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is the hallmark of a saturated hydrocarbon?
A)a hydrocarbon where carbon has only a single bond to two or more other carbons
B)a hydrocarbon that is completely dissolved in water
C)a hydrocarbon where carbon has multiple bonds to one or more other carbons
D)any molecule that is completely dissolved in a hydrocarbon solvent
E)none of the above
A)a hydrocarbon where carbon has only a single bond to two or more other carbons
B)a hydrocarbon that is completely dissolved in water
C)a hydrocarbon where carbon has multiple bonds to one or more other carbons
D)any molecule that is completely dissolved in a hydrocarbon solvent
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
43
A single carbon atom is bonded to only one other carbon atom and also two hydrogen atoms. How many bonds are formed between the two carbon atoms?
A)2
B)1
C)3
D)4
E)none of the above
A)2
B)1
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
44
What is the chemical formula of the following structure? 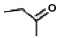
A) (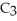
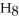 O)
O)
B)(
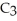
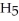 O)
O)
C) (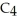
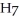 O)
O)
D) (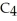
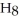 O)
O)

A) (
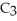
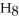 O)
O)B)(
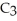
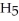 O)
O)C) (
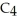
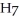 O)
O)D) (
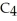
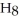 O)
O)
Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
45
Do heavier hydrocarbons tend to produce more or less carbon dioxide upon combustion compared to lighter hydrocarbons? Why?
A)Heavier hydrocarbons produce less carbon dioxide because by percentage there is less carbon in their molecular structure.
B)Heavier hydrocarbons produce more carbon dioxide because they have a greater proportion of carbon in their molecular structure.
C)Heavier hydrocarbons produce less carbon dioxide because they tend to produce both carbon monoxide and carbon trioxide in addition to carbon dioxide when they combust.
D)Heavier hydrocarbons produce the same amount carbon dioxide as lighter hydrocarbons because complete combustion always results in the same two compounds,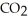 and
and 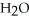 .
.
A)Heavier hydrocarbons produce less carbon dioxide because by percentage there is less carbon in their molecular structure.
B)Heavier hydrocarbons produce more carbon dioxide because they have a greater proportion of carbon in their molecular structure.
C)Heavier hydrocarbons produce less carbon dioxide because they tend to produce both carbon monoxide and carbon trioxide in addition to carbon dioxide when they combust.
D)Heavier hydrocarbons produce the same amount carbon dioxide as lighter hydrocarbons because complete combustion always results in the same two compounds,
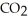 and
and 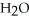 .
.
Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
46
Which number set identifies the longest chain of carbons in the following structure? 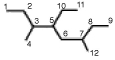
A)1, 2, 3, 5, 10, 11, 6, 7, 8, 9
B)9, 8, 7, 12, 6, 5, 3, 2, 1
C)9, 8, 7, 6, 5, 3, 2, 1
D)9, 8, 7, 6, 5, 10, 11

A)1, 2, 3, 5, 10, 11, 6, 7, 8, 9
B)9, 8, 7, 12, 6, 5, 3, 2, 1
C)9, 8, 7, 6, 5, 3, 2, 1
D)9, 8, 7, 6, 5, 10, 11

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
47
Hydrocarbons with a carbon-carbon double bond are known as ________.
A)alkanes
B)alkenes
C)alkynes
D)aromatics
A)alkanes
B)alkenes
C)alkynes
D)aromatics

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is a saturated molecule? 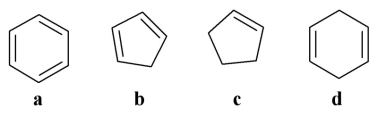
A)a
B)b
C)c
D)d
E)none of the above

A)a
B)b
C)c
D)d
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the above structures might be wrongly named 1, 4-diethylbutane?
A)a
B)b
C)c
D)All of the above have the same octane rating.
E)None of the above are octane.
A)a
B)b
C)c
D)All of the above have the same octane rating.
E)None of the above are octane.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
50
Shown below on the left is the structure of 2-methylpentane. What is the name of the structure on the right, labeled (b)? 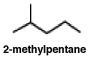 (b)
(b) 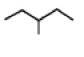
A)2-methylpentane
B)3-methylpentane
C)isopentylmethane
D)2-ethylbutane
 (b)
(b) 
A)2-methylpentane
B)3-methylpentane
C)isopentylmethane
D)2-ethylbutane

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following does not describe the number of other atoms a carbon atom can be bonded to?
A)only 1
B)only 2
C)only 3
D)only 4
E)only 5
A)only 1
B)only 2
C)only 3
D)only 4
E)only 5

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the above structures is 2, 5-dimethyloctane?
A)a
B)b
C)c
D)None of the above
A)a
B)b
C)c
D)None of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
53
A single carbon branch within a hydrocarbon is referred to as a methyl group, while a two carbon branch is referred to as an ethyl group. What then is the formal name for the structure shown below? 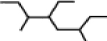
A)3-methyl-4-ethyl-6-methyloctane
B)3,6-dimethyl-4-ethyloctane
C)2-ethyl-4-ethyl-5-methylheptane
D)2,4-diethyl-5-methylheptane

A)3-methyl-4-ethyl-6-methyloctane
B)3,6-dimethyl-4-ethyloctane
C)2-ethyl-4-ethyl-5-methylheptane
D)2,4-diethyl-5-methylheptane

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the above structures should be named as a heptane?
A)a
B)b
C)c
D)None of the above
A)a
B)b
C)c
D)None of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
55
A single carbon atom is bonded to two other carbon atom and also two hydrogen atoms. How many bonds are formed between the each carbon atoms?
A)2
B)1
C)3
D)4
E)none of the above
A)2
B)1
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
56
A single carbon atom is bonded to only one carbon and only one hydrogen atom. How many bonds are formed between the attached carbon atoms?
A)2
B)1
C)3
D)4
E)none of the above
A)2
B)1
C)3
D)4
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
57
The temperatures in a fractionating tower at an oil refinery are important, but so are the pressures. Where might the pressure in a fractional distillation tower be greatest, at the bottom or at the top? Defend your answer.
A)The pressure is greatest at the bottom because the higher temperature means a greater number of vaporized molecules.
B)The pressure is greatest at the top because the higher temperature means a greater number of vaporized molecules.
C)The pressure is greatest at the bottom because the lower temperature means a greater number of vaporized molecules.
D)The pressure is greatest at the top because the lower temperature means a greater number of vaporized molecules.
A)The pressure is greatest at the bottom because the higher temperature means a greater number of vaporized molecules.
B)The pressure is greatest at the top because the higher temperature means a greater number of vaporized molecules.
C)The pressure is greatest at the bottom because the lower temperature means a greater number of vaporized molecules.
D)The pressure is greatest at the top because the lower temperature means a greater number of vaporized molecules.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
58
What is the chemical formula for the following structure? 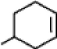
A)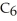
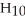
B)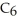
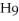
C)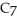
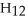
D)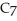
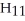

A)
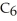
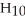
B)
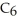
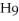
C)
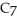
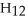
D)
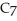
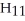

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the above structures is 2, 2, 4-trimethylpentane?
A)a
B)b
C)c
D)None of the above
A)a
B)b
C)c
D)None of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following does not contain a double bond? 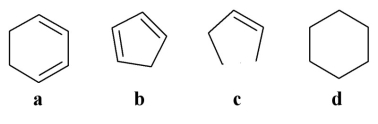
A)a
B)b
C)c
D)d
E)All of the above are unsaturated.

A)a
B)b
C)c
D)d
E)All of the above are unsaturated.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
61
Heteroatoms make a difference in the physical and chemical properties of an organic molecule because ________.
A)they add extra mass to the hydrocarbon structure
B)each heteroatom has its own characteristic chemistry
C)they can enhance the polarity of the organic molecule
D)all of the above
A)they add extra mass to the hydrocarbon structure
B)each heteroatom has its own characteristic chemistry
C)they can enhance the polarity of the organic molecule
D)all of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
62
Formaldehyde is a toxic preservative with the following chemical formula: H2C  O Which of the following compounds would best serve as a starting point for its production?
O Which of the following compounds would best serve as a starting point for its production?
A)H3COH
B)H2C CH2
CH2
C)H2O
D)CH3CH2OH
E)none of the above
 O Which of the following compounds would best serve as a starting point for its production?
O Which of the following compounds would best serve as a starting point for its production?A)H3COH
B)H2C
 CH2
CH2C)H2O
D)CH3CH2OH
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
63
What is a functional group?
A)a set of atoms bonded together that give molecules containing them similar properties
B)a group that adds function
C)a group of hydrocarbons that can be used to make new materials
D)a set of molecules that are grouped together to form a functional material
E)none of the above
A)a set of atoms bonded together that give molecules containing them similar properties
B)a group that adds function
C)a group of hydrocarbons that can be used to make new materials
D)a set of molecules that are grouped together to form a functional material
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
64
List the following compounds in order of least oxidized to most oxidized 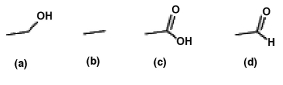
A)a < b < c < d
B)b < a < c < d
C)c < d < a < b
D)b < a < d < c

A)a < b < c < d
B)b < a < c < d
C)c < d < a < b
D)b < a < d < c

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
65
Another name for the "alcohol" of alcoholic beverages is ________.
A)ethylene
B)hydroxyethane
C)ethyl acetate
D)gylcerol
A)ethylene
B)hydroxyethane
C)ethyl acetate
D)gylcerol

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
66
How many different isomers of 3-methyl-2-pentene are there?
A)5
B)2
C)3
D)4
A)5
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following is a heteroatom?
A)O
B)C
C)H
D)A and B
E)all of the above
A)O
B)C
C)H
D)A and B
E)all of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
68
Carbon-carbon single bonds can rotate while carbon-carbon double bonds cannot rotate. How many different structures are shown below. 
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
69
The class of organic compounds without a functional group are the ________.
A)saturated hydrocarbons
B)carboyxlic acids
C)alcohols
D)esters
A)saturated hydrocarbons
B)carboyxlic acids
C)alcohols
D)esters

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
70
The "alcohol" of alcoholic beverages is ________.
A)ethyl amine
B)ethano
C)ethyl acetate
D)phenol
A)ethyl amine
B)ethano
C)ethyl acetate
D)phenol

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the above sections of this molecule would be considered to be the aromatic portion of the molecule?
A)a
B)b
C)c
D)a and b
E)all of the above
A)a
B)b
C)c
D)a and b
E)all of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
72
Correctly identify the following functional groups in this organic molecule-amide, ester, ketone, ether, alcohol, aldehyde, amine. 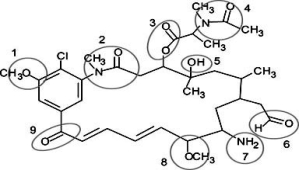
A)1 = ether, 3 = ester, 6 = aldehyde, 9 = alcohol
B)2 = amide, 4 = ester, 7 = amine, 8 = ether
C)1 = ester, 5 = alcohol, 8 = ether, 9 = ketone
D)2 = amide, 6 = aldehyde, 7 = amine, 8 = ether

A)1 = ether, 3 = ester, 6 = aldehyde, 9 = alcohol
B)2 = amide, 4 = ester, 7 = amine, 8 = ether
C)1 = ester, 5 = alcohol, 8 = ether, 9 = ketone
D)2 = amide, 6 = aldehyde, 7 = amine, 8 = ether

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following molecules is an alcohol? 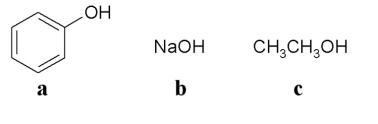
A)a
B)b
C)c
D)a or b
E)a or c
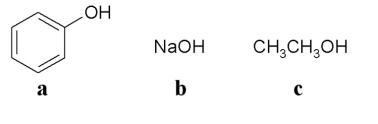
A)a
B)b
C)c
D)a or b
E)a or c

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
74
If you were throwing a party, which of these compounds would probably be on the beverage list?
A)CH3CH2OH
B)CH3CH2CH3
C)CH3OH
D)CH3CH CH2
CH2
E)Milk, it does the body good.
A)CH3CH2OH
B)CH3CH2CH3
C)CH3OH
D)CH3CH
 CH2
CH2E)Milk, it does the body good.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the above sections of this molecule would be considered a saturated portion of the molecule?
A)a
B)b
C)c
D)a and b
E)all of the above
A)a
B)b
C)c
D)a and b
E)all of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the above sections of this molecule would be considered an unsaturated portion of the molecule?
A)a
B)b
C)c
D)a and b
E)all of the above
A)a
B)b
C)c
D)a and b
E)all of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is not a heteroatom?
A)O
B)N
C)P
D)A and B
E)All of the above are heteroatoms.
A)O
B)N
C)P
D)A and B
E)All of the above are heteroatoms.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following statements best describes the effect of heteroatoms on hydrocarbons?
A)Heteroatoms greatly alter the chemical and physical properties of a hydrocarbon.
B)Heteroatoms have little effect on the chemical and physical properties of a hydrocarbon.
C)Heteroatoms have very similar properties to hydrocarbons.
D)The properties of the heteroatom compounds depend only on the atom, not how it is bonded to the hydrocarbon.
E)none of the above
A)Heteroatoms greatly alter the chemical and physical properties of a hydrocarbon.
B)Heteroatoms have little effect on the chemical and physical properties of a hydrocarbon.
C)Heteroatoms have very similar properties to hydrocarbons.
D)The properties of the heteroatom compounds depend only on the atom, not how it is bonded to the hydrocarbon.
E)none of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the structures shown below are 3-methyl-2-pentene, where "2" indicates the placement of the double bond?  a b c d
a b c d
A)b and d are 3-methyl-2-pentene.
B)b, c, and d are 3-methyl-2-pentene.
C)a and b are 3-methyl-2-pentene.
D)All of them are 3-methyl-2-pentene.
 a b c d
a b c dA)b and d are 3-methyl-2-pentene.
B)b, c, and d are 3-methyl-2-pentene.
C)a and b are 3-methyl-2-pentene.
D)All of them are 3-methyl-2-pentene.

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following is an aromatic molecule? 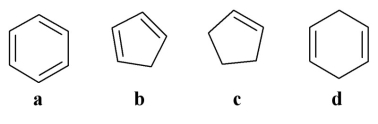
A)a
B)b
C)c
D)d
E)all of the above

A)a
B)b
C)c
D)d
E)all of the above

Unlock Deck
Unlock for access to all 162 flashcards in this deck.
Unlock Deck
k this deck



