Deck 14: Chemical Kinetics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/134
Play
Full screen (f)
Deck 14: Chemical Kinetics
1
The reaction A → B is first order in [A]. Consider the following data. ![<strong>The reaction A → B is first order in [A]. Consider the following data. The concentration of A is ________ M after 40.0 s.</strong> A)1.3 × 10<sup>-2</sup> B)1.2 C)0.17 D)3.5 × 10<sup>-4</sup> E)0.025](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_ed9c_a2ab_f3d6931b79e5_TB2701_00_TB2701_00_TB2701_00.jpg)
The concentration of A is ________ M after 40.0 s.
A)1.3 × 10-2
B)1.2
C)0.17
D)3.5 × 10-4
E)0.025
![<strong>The reaction A → B is first order in [A]. Consider the following data. The concentration of A is ________ M after 40.0 s.</strong> A)1.3 × 10<sup>-2</sup> B)1.2 C)0.17 D)3.5 × 10<sup>-4</sup> E)0.025](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_ed9c_a2ab_f3d6931b79e5_TB2701_00_TB2701_00_TB2701_00.jpg)
The concentration of A is ________ M after 40.0 s.
A)1.3 × 10-2
B)1.2
C)0.17
D)3.5 × 10-4
E)0.025
1.3 × 10-2
2
The data in the table below were obtained for the reaction:
A + B → P![<strong>The data in the table below were obtained for the reaction: A + B → P The rate law for this reaction is rate = ________.</strong> A)k[A][B] B)k[P] C)k[A]<sup>2</sup>[B] D)k[A]<sup>2</sup>[B]<sup>2</sup> E)k[A]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_0333_a2ab_bf7bd09af565_TB2701_00_TB2701_00.jpg)
The rate law for this reaction is rate = ________.
A)k[A][B]
B)k[P]
C)k[A]2[B]
D)k[A]2[B]2
E)k[A]2
A + B → P
![<strong>The data in the table below were obtained for the reaction: A + B → P The rate law for this reaction is rate = ________.</strong> A)k[A][B] B)k[P] C)k[A]<sup>2</sup>[B] D)k[A]<sup>2</sup>[B]<sup>2</sup> E)k[A]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_0333_a2ab_bf7bd09af565_TB2701_00_TB2701_00.jpg)
The rate law for this reaction is rate = ________.
A)k[A][B]
B)k[P]
C)k[A]2[B]
D)k[A]2[B]2
E)k[A]2
k[A]2
3
Which one of the following is not a valid expression for the rate of the reaction below? 4NH3 + 7O2 → 4NO2 + 6H2O
A)-
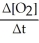
B)
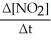
C)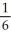
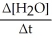
D)-
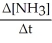
E)All of the above are valid expressions of the reaction rate.
A)-

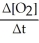
B)

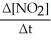
C)
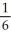
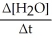
D)-

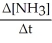
E)All of the above are valid expressions of the reaction rate.
All of the above are valid expressions of the reaction rate.
4
A burning splint will burn more vigorously in pure oxygen than in air because ________.
A)oxygen is a reactant in combustion and concentration of oxygen is higher in pure oxygen than is in air
B)oxygen is a catalyst for combustion
C)oxygen is a product of combustion
D)nitrogen is a product of combustion and the system reaches equilibrium at a lower temperature
E)nitrogen is a reactant in combustion and its low concentration in pure oxygen catalyzes the combustion
A)oxygen is a reactant in combustion and concentration of oxygen is higher in pure oxygen than is in air
B)oxygen is a catalyst for combustion
C)oxygen is a product of combustion
D)nitrogen is a product of combustion and the system reaches equilibrium at a lower temperature
E)nitrogen is a reactant in combustion and its low concentration in pure oxygen catalyzes the combustion

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
5
The reaction A (aq) → B (aq)is first order in [A]. A solution is prepared with [A] = 1.22 M. The following data are obtained as the reaction proceeds: ![<strong>The reaction A (aq) → B (aq)is first order in [A]. A solution is prepared with [A] = 1.22 M. The following data are obtained as the reaction proceeds: The rate constant for this reaction is ________ s<sup>-1</sup>.</strong> A)0.23 B)1.0 C)0.17 D)0.12 E)-0.12](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f62_14ad_a2ab_6f47494b9eef_TB2701_00.jpg) The rate constant for this reaction is ________ s-1.
The rate constant for this reaction is ________ s-1.
A)0.23
B)1.0
C)0.17
D)0.12
E)-0.12
![<strong>The reaction A (aq) → B (aq)is first order in [A]. A solution is prepared with [A] = 1.22 M. The following data are obtained as the reaction proceeds: The rate constant for this reaction is ________ s<sup>-1</sup>.</strong> A)0.23 B)1.0 C)0.17 D)0.12 E)-0.12](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f62_14ad_a2ab_6f47494b9eef_TB2701_00.jpg) The rate constant for this reaction is ________ s-1.
The rate constant for this reaction is ________ s-1.A)0.23
B)1.0
C)0.17
D)0.12
E)-0.12

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
6
The data in the table below were obtained for the reaction:
2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1)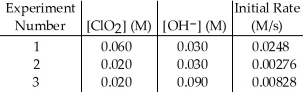
What is the order of the reaction with respect to OH-?
A)0
B)1
C)2
D)3
E)4
2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1)

What is the order of the reaction with respect to OH-?
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
7
The reaction A → B is first order in [A]. Consider the following data. ![<strong>The reaction A → B is first order in [A]. Consider the following data. The rate constant for this reaction is ________ s<sup>-1</sup>.</strong> A)6.9 × 10<sup>-2</sup> B)3.0 × 10<sup>-2</sup> C)14 D)0.46 E)4.0 × 10<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_ed9c_a2ab_f3d6931b79e5_TB2701_00_TB2701_00_TB2701_00.jpg)
The rate constant for this reaction is ________ s-1.
A)6.9 × 10-2
B)3.0 × 10-2
C)14
D)0.46
E)4.0 × 102
![<strong>The reaction A → B is first order in [A]. Consider the following data. The rate constant for this reaction is ________ s<sup>-1</sup>.</strong> A)6.9 × 10<sup>-2</sup> B)3.0 × 10<sup>-2</sup> C)14 D)0.46 E)4.0 × 10<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_ed9c_a2ab_f3d6931b79e5_TB2701_00_TB2701_00_TB2701_00.jpg)
The rate constant for this reaction is ________ s-1.
A)6.9 × 10-2
B)3.0 × 10-2
C)14
D)0.46
E)4.0 × 102

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
8
The reaction A → B is first order in [A]. Consider the following data. ![<strong>The reaction A → B is first order in [A]. Consider the following data. The rate constant of a first-order process that has a half-life of 3.50 min is ________ s<sup>-1</sup>.</strong> A)0.693 B)1.65 × 10<sup>-2</sup> C)1.98 D)0.198 E)3.30 ×10<sup>-3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_ed9c_a2ab_f3d6931b79e5_TB2701_00_TB2701_00_TB2701_00.jpg)
The rate constant of a first-order process that has a half-life of 3.50 min is ________ s-1.
A)0.693
B)1.65 × 10-2
C)1.98
D)0.198
E)3.30 ×10-3
![<strong>The reaction A → B is first order in [A]. Consider the following data. The rate constant of a first-order process that has a half-life of 3.50 min is ________ s<sup>-1</sup>.</strong> A)0.693 B)1.65 × 10<sup>-2</sup> C)1.98 D)0.198 E)3.30 ×10<sup>-3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_ed9c_a2ab_f3d6931b79e5_TB2701_00_TB2701_00_TB2701_00.jpg)
The rate constant of a first-order process that has a half-life of 3.50 min is ________ s-1.
A)0.693
B)1.65 × 10-2
C)1.98
D)0.198
E)3.30 ×10-3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
9
The rate law of a reaction is rate = k[D][X]. The units of the rate constant are ________.
A)mol L-1s-1
B)L mol-1s-1
C)mol2 L-2s-1
D)mol L-1s-2
E)L2 mol-2s-1
A)mol L-1s-1
B)L mol-1s-1
C)mol2 L-2s-1
D)mol L-1s-2
E)L2 mol-2s-1

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
10
The reaction 2NO2 → 2NO + O2
Follows second-order kinetics. At 300 °C, [NO2] drops from 0.0100 M to 0.00650 M in 100.0 s. The rate constant for the reaction is ________ M-1s-1.
A)0.096
B)0.65
C)0.81
D)1.2
E)0.54
Follows second-order kinetics. At 300 °C, [NO2] drops from 0.0100 M to 0.00650 M in 100.0 s. The rate constant for the reaction is ________ M-1s-1.
A)0.096
B)0.65
C)0.81
D)1.2
E)0.54

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
11
One difference between first- and second-order reactions is that ________.
A)the half-life of a first-order reaction does not depend on [A]0; the half-life of a second-order reaction does depend on [A]0
B)the rate of both first-order and second-order reactions do not depend on reactant concentrations
C)the rate of a first-order reaction depends on reactant concentrations; the rate of a second-order reaction does not depend on reactant concentrations
D)a first-order reaction can be catalyzed; a second-order reaction cannot be catalyzed
E)None of the above are true.
A)the half-life of a first-order reaction does not depend on [A]0; the half-life of a second-order reaction does depend on [A]0
B)the rate of both first-order and second-order reactions do not depend on reactant concentrations
C)the rate of a first-order reaction depends on reactant concentrations; the rate of a second-order reaction does not depend on reactant concentrations
D)a first-order reaction can be catalyzed; a second-order reaction cannot be catalyzed
E)None of the above are true.

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
12
The reaction CH3-N≡C → CH3-C≡N
Is a first-order reaction. At 230.3 °C, k = 6.29 × 10-4s-1. If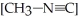 is 1.00 × 10-3 initially,
is 1.00 × 10-3 initially, 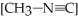 is ________ after 1.000 × 103 s.
is ________ after 1.000 × 103 s.
A)5.33 × 10-4
B)2.34 × 10-4
C)1.88 × 10-3
D)4.27 × 10-3
E)1.00 × 10-6
Is a first-order reaction. At 230.3 °C, k = 6.29 × 10-4s-1. If
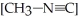 is 1.00 × 10-3 initially,
is 1.00 × 10-3 initially, 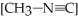 is ________ after 1.000 × 103 s.
is ________ after 1.000 × 103 s.A)5.33 × 10-4
B)2.34 × 10-4
C)1.88 × 10-3
D)4.27 × 10-3
E)1.00 × 10-6

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
13
Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?
A)![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_9f77_a2ab_7b4947523bf7_TB2701_11.jpg)
B)![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_9f78_a2ab_effb6f5de32a_TB2701_11.jpg)
C)![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_9f79_a2ab_6192b6ed8d0f_TB2701_11.jpg)
D)![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_c68a_a2ab_87bd4641e38c_TB2701_11.jpg)
E)![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_c68b_a2ab_31fe9e7fda13_TB2701_11.jpg)
A)
![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_9f77_a2ab_7b4947523bf7_TB2701_11.jpg)
B)
![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_9f78_a2ab_effb6f5de32a_TB2701_11.jpg)
C)
![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_9f79_a2ab_6192b6ed8d0f_TB2701_11.jpg)
D)
![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_c68a_a2ab_87bd4641e38c_TB2701_11.jpg)
E)
![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f61_c68b_a2ab_31fe9e7fda13_TB2701_11.jpg)

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
14
Of the following, all are valid units for a reaction rate except ________.
A)mol/L
B)M/s
C)mol/hr
D)g/s
E)mol/L-hr
A)mol/L
B)M/s
C)mol/hr
D)g/s
E)mol/L-hr

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
15
The following reaction is second order in [A] and the rate constant is 0.025 M-1s-1: A → B
The concentration of A was 0.65 M at 33 s. The initial concentration of A was ________ M.
A)2.4
B)0.27
C)0.24
D)1.4
E)1.2 × 10-2
The concentration of A was 0.65 M at 33 s. The initial concentration of A was ________ M.
A)2.4
B)0.27
C)0.24
D)1.4
E)1.2 × 10-2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
16
The data in the table below were obtained for the reaction:
2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1)
What is the order of the reaction with respect to ClO2?
A)1
B)0
C)2
D)3
E)4
2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1)

What is the order of the reaction with respect to ClO2?
A)1
B)0
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
17
The data in the table below were obtained for the reaction:
2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1)
What is the overall order of the reaction?
A)4
B)0
C)1
D)2
E)3
2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1)

What is the overall order of the reaction?
A)4
B)0
C)1
D)2
E)3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
18
The data in the table below were obtained for the reaction:
A + B → P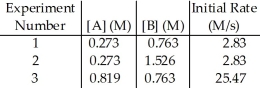
The magnitude of the rate constant is ________.
A)38.0
B)0.278
C)13.2
D)42.0
E)2.21
A + B → P

The magnitude of the rate constant is ________.
A)38.0
B)0.278
C)13.2
D)42.0
E)2.21

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
19
The data in the table below were obtained for the reaction:
2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1)
What is the magnitude of the rate constant for the reaction?
A)1.15 × 104
B)4.6
C)230
D)115
E)713
2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1)

What is the magnitude of the rate constant for the reaction?
A)1.15 × 104
B)4.6
C)230
D)115
E)713

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
20
Under constant conditions, the half-life of a first-order reaction ________.
A)is the time necessary for the reactant concentration to drop to half its original value
B)is constant
C)can be calculated from the reaction rate constant
D)does not depend on the initial reactant concentration
E)All of the above are correct.
A)is the time necessary for the reactant concentration to drop to half its original value
B)is constant
C)can be calculated from the reaction rate constant
D)does not depend on the initial reactant concentration
E)All of the above are correct.

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
21
A catalyst can increase the rate of a reaction ________.
A)by changing the value of the frequency factor (A)
B)by increasing the overall activation energy (Ea)of the reaction
C)by lowering the activation energy of the reverse reaction
D)by providing an alternative pathway with a lower activation energy
E)All of these are ways that a catalyst might act to increase the rate of reaction.
A)by changing the value of the frequency factor (A)
B)by increasing the overall activation energy (Ea)of the reaction
C)by lowering the activation energy of the reverse reaction
D)by providing an alternative pathway with a lower activation energy
E)All of these are ways that a catalyst might act to increase the rate of reaction.

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is true?
A)If we know that a reaction is an elementary reaction, then we know its rate law.
B)The rate-determining step of a reaction is the rate of the fastest elementary step of its mechanism.
C)Since intermediate compounds can be formed, the chemical equations for the elementary reactions in a multistep mechanism do not always have to add to give the chemical equation of the overall process.
D)In a reaction mechanism, an intermediate is identical to an activated complex.
E)All of the above statements are true.
A)If we know that a reaction is an elementary reaction, then we know its rate law.
B)The rate-determining step of a reaction is the rate of the fastest elementary step of its mechanism.
C)Since intermediate compounds can be formed, the chemical equations for the elementary reactions in a multistep mechanism do not always have to add to give the chemical equation of the overall process.
D)In a reaction mechanism, an intermediate is identical to an activated complex.
E)All of the above statements are true.

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
23
For the elementary reaction NO3 + CO → NO2 + CO2
The molecularity of the reaction is ________, and the rate law is![<strong>For the elementary reaction NO<sub>3</sub> + CO → NO<sub>2</sub> + CO<sub>2</sub> The molecularity of the reaction is ________, and the rate law is </strong> A)2, k[NO<sub>3</sub>][CO] B)4, k[NO<sub>3</sub>][CO][NO<sub>2</sub>][CO<sub>2</sub>] C)2, k[NO<sub>2</sub>][CO<sub>2</sub>] D)2, k[NO<sub>3</sub>][CO]/[NO<sub>2</sub>][CO<sub>2</sub>] E)4, k[NO<sub>2</sub>][CO<sub>2</sub>]/[NO<sub>3</sub>][CO]](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f62_b0f3_a2ab_1d7683edb257_TB2701_11.jpg)
A)2, k[NO3][CO]
B)4, k[NO3][CO][NO2][CO2]
C)2, k[NO2][CO2]
D)2, k[NO3][CO]/[NO2][CO2]
E)4, k[NO2][CO2]/[NO3][CO]
The molecularity of the reaction is ________, and the rate law is
![<strong>For the elementary reaction NO<sub>3</sub> + CO → NO<sub>2</sub> + CO<sub>2</sub> The molecularity of the reaction is ________, and the rate law is </strong> A)2, k[NO<sub>3</sub>][CO] B)4, k[NO<sub>3</sub>][CO][NO<sub>2</sub>][CO<sub>2</sub>] C)2, k[NO<sub>2</sub>][CO<sub>2</sub>] D)2, k[NO<sub>3</sub>][CO]/[NO<sub>2</sub>][CO<sub>2</sub>] E)4, k[NO<sub>2</sub>][CO<sub>2</sub>]/[NO<sub>3</sub>][CO]](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f62_b0f3_a2ab_1d7683edb257_TB2701_11.jpg)
A)2, k[NO3][CO]
B)4, k[NO3][CO][NO2][CO2]
C)2, k[NO2][CO2]
D)2, k[NO3][CO]/[NO2][CO2]
E)4, k[NO2][CO2]/[NO3][CO]

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
24
________ are used in automotive catalytic converters.
A)Heterogeneous catalysts
B)Homogeneous catalysts
C)Enzymes
D)Noble gases
E)Nonmetal oxides
A)Heterogeneous catalysts
B)Homogeneous catalysts
C)Enzymes
D)Noble gases
E)Nonmetal oxides

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
25
As the temperature of a reaction is increased, the rate of the reaction increases because the ________.
A)reactant molecules collide less frequently
B)reactant molecules collide more frequently and with greater energy per collision
C)activation energy is lowered
D)reactant molecules collide less frequently and with greater energy per collision
E)reactant molecules collide more frequently with less energy per collision
A)reactant molecules collide less frequently
B)reactant molecules collide more frequently and with greater energy per collision
C)activation energy is lowered
D)reactant molecules collide less frequently and with greater energy per collision
E)reactant molecules collide more frequently with less energy per collision

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
26
The decomposition of N2O5 in solution in carbon tetrachloride proceeds via the reaction 2N2O5 (soln) → 4NO2 (soln) + O2 (soln)
The reaction is first order and has a rate constant of 4.82 × 10-3 s-1 at 64 °C. The rate law for the reaction is rate = ________.
A)k[N2O5]2
B)k![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub> in solution in carbon tetrachloride proceeds via the reaction 2N<sub>2</sub>O<sub>5 </sub>(soln) → 4NO<sub>2 </sub>(soln) + O<sub>2 </sub>(soln) The reaction is first order and has a rate constant of 4.82 × 10<sup>-3</sup> s<sup>-1</sup> at 64 °C. The rate law for the reaction is rate = ________.</strong> A)k[N<sub>2</sub>O<sub>5</sub>]<sup>2</sup> B)k C)k[N<sub>2</sub>O<sub>5</sub>] D)k E)2k[N<sub>2</sub>O<sub>5</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f62_3bbf_a2ab_9bd6aa4c473b_TB2701_11.jpg)
C)k[N2O5]
D)k![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub> in solution in carbon tetrachloride proceeds via the reaction 2N<sub>2</sub>O<sub>5 </sub>(soln) → 4NO<sub>2 </sub>(soln) + O<sub>2 </sub>(soln) The reaction is first order and has a rate constant of 4.82 × 10<sup>-3</sup> s<sup>-1</sup> at 64 °C. The rate law for the reaction is rate = ________.</strong> A)k[N<sub>2</sub>O<sub>5</sub>]<sup>2</sup> B)k C)k[N<sub>2</sub>O<sub>5</sub>] D)k E)2k[N<sub>2</sub>O<sub>5</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f62_3bc0_a2ab_29471ab7fcc3_TB2701_11.jpg)
E)2k[N2O5]
The reaction is first order and has a rate constant of 4.82 × 10-3 s-1 at 64 °C. The rate law for the reaction is rate = ________.
A)k[N2O5]2
B)k
![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub> in solution in carbon tetrachloride proceeds via the reaction 2N<sub>2</sub>O<sub>5 </sub>(soln) → 4NO<sub>2 </sub>(soln) + O<sub>2 </sub>(soln) The reaction is first order and has a rate constant of 4.82 × 10<sup>-3</sup> s<sup>-1</sup> at 64 °C. The rate law for the reaction is rate = ________.</strong> A)k[N<sub>2</sub>O<sub>5</sub>]<sup>2</sup> B)k C)k[N<sub>2</sub>O<sub>5</sub>] D)k E)2k[N<sub>2</sub>O<sub>5</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f62_3bbf_a2ab_9bd6aa4c473b_TB2701_11.jpg)
C)k[N2O5]
D)k
![<strong>The decomposition of N<sub>2</sub>O<sub>5</sub> in solution in carbon tetrachloride proceeds via the reaction 2N<sub>2</sub>O<sub>5 </sub>(soln) → 4NO<sub>2 </sub>(soln) + O<sub>2 </sub>(soln) The reaction is first order and has a rate constant of 4.82 × 10<sup>-3</sup> s<sup>-1</sup> at 64 °C. The rate law for the reaction is rate = ________.</strong> A)k[N<sub>2</sub>O<sub>5</sub>]<sup>2</sup> B)k C)k[N<sub>2</sub>O<sub>5</sub>] D)k E)2k[N<sub>2</sub>O<sub>5</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f62_3bc0_a2ab_29471ab7fcc3_TB2701_11.jpg)
E)2k[N2O5]

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
27
Which energy difference in the energy profile below corresponds to the activation energy for the forward reaction? 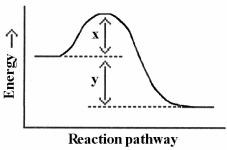
A)x
B)y
C)x + y
D)x - y
E)y - x

A)x
B)y
C)x + y
D)x - y
E)y - x

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
28
In the Arrhenius equation, k = Ae-Ea/RT
________ is the frequency factor.
A)k
B)A
C)e
D)Ea
E)R
________ is the frequency factor.
A)k
B)A
C)e
D)Ea
E)R

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
29
In general, as temperature goes up, reaction rate ________.
A)goes up if the reaction is exothermic
B)goes up if the reaction is endothermic
C)goes up regardless of whether the reaction is exothermic or endothermic
D)stays the same regardless of whether the reaction is exothermic or endothermic
E)stays the same if the reaction is first order
A)goes up if the reaction is exothermic
B)goes up if the reaction is endothermic
C)goes up regardless of whether the reaction is exothermic or endothermic
D)stays the same regardless of whether the reaction is exothermic or endothermic
E)stays the same if the reaction is first order

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
30
Of the following, ________ will lower the activation energy for a reaction.
A)increasing the concentrations of reactants
B)raising the temperature of the reaction
C)adding a catalyst for the reaction
D)removing products as the reaction proceeds
E)increasing the pressure
A)increasing the concentrations of reactants
B)raising the temperature of the reaction
C)adding a catalyst for the reaction
D)removing products as the reaction proceeds
E)increasing the pressure

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
31
At elevated temperatures, methylisonitrile (CH3NC)isomerizes to acetonitrile (CH3CN): CH3NC (g) → CH3CN (g)
The reaction is first order in methylisonitrile. The attached graph shows data for the reaction obtained at 198.9 °C.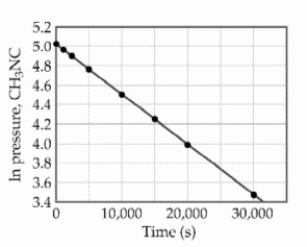 The rate constant for the reaction is ________ s-1.
The rate constant for the reaction is ________ s-1.
A)-1.9 × 104
B)+1.9 × 104
C)-5.2 × 10-5
D)+5.2 × 10-5
E)+6.2
The reaction is first order in methylisonitrile. The attached graph shows data for the reaction obtained at 198.9 °C.
 The rate constant for the reaction is ________ s-1.
The rate constant for the reaction is ________ s-1.A)-1.9 × 104
B)+1.9 × 104
C)-5.2 × 10-5
D)+5.2 × 10-5
E)+6.2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
32
The rate of a reaction depends on ________.
A)collision frequency
B)collision energy
C)collision orientation
D)all of the above
E)none of the above
A)collision frequency
B)collision energy
C)collision orientation
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
33
The primary source of the specificity of enzymes is ________.
A)their polarity, which matches that of their specific substrate
B)their delocalized electron cloud
C)their bonded transition metal, which is specific to the target substrate
D)their locations within the cell
E)their shape, which relates to the lock-and-key model
A)their polarity, which matches that of their specific substrate
B)their delocalized electron cloud
C)their bonded transition metal, which is specific to the target substrate
D)their locations within the cell
E)their shape, which relates to the lock-and-key model

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
34
The mechanism for formation of the product X is: A + B → C + D (slow)
B + D → X (fast)
The intermediate reactant in the reaction is ________.
A)A
B)B
C)C
D)D
E)X
B + D → X (fast)
The intermediate reactant in the reaction is ________.
A)A
B)B
C)C
D)D
E)X

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
35
At elevated temperatures, methylisonitrile (CH3NC)isomerizes to acetonitrile (CH3CN): CH3NC (g) → CH3CN (g)
The dependence of the rate constant on temperature is studied and the graph below is prepared from the results.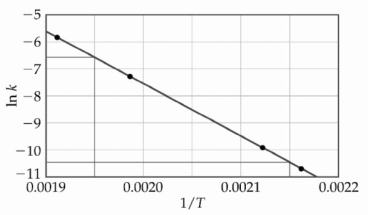 The energy of activation of this reaction is ________ kJ/mol.
The energy of activation of this reaction is ________ kJ/mol.
A)160
B)1.6 × 105
C)4.4 × 10-7
D)4.4 × 10-4
E)1.9 × 104
The dependence of the rate constant on temperature is studied and the graph below is prepared from the results.
 The energy of activation of this reaction is ________ kJ/mol.
The energy of activation of this reaction is ________ kJ/mol.A)160
B)1.6 × 105
C)4.4 × 10-7
D)4.4 × 10-4
E)1.9 × 104

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
36
In general, as activation energy increases, reaction rate ________.
A)goes down if the reaction is exothermic
B)goes down if the reaction is endothermic
C)stays the same regardless of whether the reaction is exothermic or endothermic
D)goes down regardless of whether the reaction is exothermic or endothermic
E)none of the above
A)goes down if the reaction is exothermic
B)goes down if the reaction is endothermic
C)stays the same regardless of whether the reaction is exothermic or endothermic
D)goes down regardless of whether the reaction is exothermic or endothermic
E)none of the above

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
37
The enzyme nitrogenase converts ________ into ________.
A)ammonia, urea
B)CO and unburned hydrocarbons, H2O and CO2
C)nitrogen, ammonia
D)nitrogen oxides, N2 and O2
E)nitroglycerine, nitric acid, and glycerine
A)ammonia, urea
B)CO and unburned hydrocarbons, H2O and CO2
C)nitrogen, ammonia
D)nitrogen oxides, N2 and O2
E)nitroglycerine, nitric acid, and glycerine

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
38
In the energy profile of a reaction, the species that exists at the maximum on the curve is called the ________.
A)product
B)activated complex
C)activation energy
D)enthalpy of reaction
E)atomic state
A)product
B)activated complex
C)activation energy
D)enthalpy of reaction
E)atomic state

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
39
The rate law of the overall reaction A + B → C
Is rate = k[A]2. Which of the following will not increase the rate of the reaction?
A)increasing the concentration of reactant A
B)increasing the concentration of reactant B
C)increasing the temperature of the reaction
D)adding a catalyst for the reaction
E)All of these will increase the rate.
Is rate = k[A]2. Which of the following will not increase the rate of the reaction?
A)increasing the concentration of reactant A
B)increasing the concentration of reactant B
C)increasing the temperature of the reaction
D)adding a catalyst for the reaction
E)All of these will increase the rate.

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
40
A possible mechanism for the overall reaction Br2 (g) + 2NO (g) → 2NOBr (g)
Is
NO (g) + Br2 (g)![<strong>A possible mechanism for the overall reaction Br<sub>2</sub> (g) + 2NO (g) → 2NOBr (g) Is NO (g) + Br<sub>2 </sub>(g) NOBr<sub>2</sub> (g)(fast) NOBr<sub>2</sub><sub> </sub>(g) + NO (g) 2NOBr (slow) The rate law for formation of NOBr based on this mechanism is rate = ________.</strong> A)k<sub>1</sub>[NO]<sup>1/2</sup> B)k<sub>1</sub>[Br<sub>2</sub>]<sup>1/2</sup> C)(k<sub>2</sub>k<sub>1</sub>/k<sup>-1</sup>)[NO]<sup>2</sup>[Br<sub>2</sub>] D)(k<sub>1</sub>/k<sup>-1</sup>)<sup>2</sup>[NO]<sup>2</sup> E)(k<sub>2</sub>k<sub>1</sub>/k<sup>-1</sup>)[NO][Br<sub>2</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f62_b0f4_a2ab_55b0478d0c59_TB2701_11.jpg) NOBr2 (g)(fast)
NOBr2 (g)(fast)
NOBr2 (g) + NO (g)![<strong>A possible mechanism for the overall reaction Br<sub>2</sub> (g) + 2NO (g) → 2NOBr (g) Is NO (g) + Br<sub>2 </sub>(g) NOBr<sub>2</sub> (g)(fast) NOBr<sub>2</sub><sub> </sub>(g) + NO (g) 2NOBr (slow) The rate law for formation of NOBr based on this mechanism is rate = ________.</strong> A)k<sub>1</sub>[NO]<sup>1/2</sup> B)k<sub>1</sub>[Br<sub>2</sub>]<sup>1/2</sup> C)(k<sub>2</sub>k<sub>1</sub>/k<sup>-1</sup>)[NO]<sup>2</sup>[Br<sub>2</sub>] D)(k<sub>1</sub>/k<sup>-1</sup>)<sup>2</sup>[NO]<sup>2</sup> E)(k<sub>2</sub>k<sub>1</sub>/k<sup>-1</sup>)[NO][Br<sub>2</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f62_d805_a2ab_21b3bed4c917_TB2701_11.jpg) 2NOBr (slow)
2NOBr (slow)
The rate law for formation of NOBr based on this mechanism is rate = ________.
A)k1[NO]1/2
B)k1[Br2]1/2
C)(k2k1/k-1)[NO]2[Br2]
D)(k1/k-1)2[NO]2
E)(k2k1/k-1)[NO][Br2]2
Is
NO (g) + Br2 (g)
![<strong>A possible mechanism for the overall reaction Br<sub>2</sub> (g) + 2NO (g) → 2NOBr (g) Is NO (g) + Br<sub>2 </sub>(g) NOBr<sub>2</sub> (g)(fast) NOBr<sub>2</sub><sub> </sub>(g) + NO (g) 2NOBr (slow) The rate law for formation of NOBr based on this mechanism is rate = ________.</strong> A)k<sub>1</sub>[NO]<sup>1/2</sup> B)k<sub>1</sub>[Br<sub>2</sub>]<sup>1/2</sup> C)(k<sub>2</sub>k<sub>1</sub>/k<sup>-1</sup>)[NO]<sup>2</sup>[Br<sub>2</sub>] D)(k<sub>1</sub>/k<sup>-1</sup>)<sup>2</sup>[NO]<sup>2</sup> E)(k<sub>2</sub>k<sub>1</sub>/k<sup>-1</sup>)[NO][Br<sub>2</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f62_b0f4_a2ab_55b0478d0c59_TB2701_11.jpg) NOBr2 (g)(fast)
NOBr2 (g)(fast)NOBr2 (g) + NO (g)
![<strong>A possible mechanism for the overall reaction Br<sub>2</sub> (g) + 2NO (g) → 2NOBr (g) Is NO (g) + Br<sub>2 </sub>(g) NOBr<sub>2</sub> (g)(fast) NOBr<sub>2</sub><sub> </sub>(g) + NO (g) 2NOBr (slow) The rate law for formation of NOBr based on this mechanism is rate = ________.</strong> A)k<sub>1</sub>[NO]<sup>1/2</sup> B)k<sub>1</sub>[Br<sub>2</sub>]<sup>1/2</sup> C)(k<sub>2</sub>k<sub>1</sub>/k<sup>-1</sup>)[NO]<sup>2</sup>[Br<sub>2</sub>] D)(k<sub>1</sub>/k<sup>-1</sup>)<sup>2</sup>[NO]<sup>2</sup> E)(k<sub>2</sub>k<sub>1</sub>/k<sup>-1</sup>)[NO][Br<sub>2</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f62_d805_a2ab_21b3bed4c917_TB2701_11.jpg) 2NOBr (slow)
2NOBr (slow)The rate law for formation of NOBr based on this mechanism is rate = ________.
A)k1[NO]1/2
B)k1[Br2]1/2
C)(k2k1/k-1)[NO]2[Br2]
D)(k1/k-1)2[NO]2
E)(k2k1/k-1)[NO][Br2]2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
41
At elevated temperatures, methylisonitrile (CH3NC)isomerizes to acetonitrile (CH3CN): CH3NC (g) → CH3CN (g)
At the start of the experiment, there are 0.200 mol of reactant (CH3NC)and 0 mol of product (CH3CN)in the reaction vessel. After 25 min of reaction, 0.108 mol of reactant (CH3NC)remain. The average rate of decomposition of methyl isonitrile, CH3NC, in this 25 min period is ________ mol/min.
A)3.7 × 10-3
B)0.092
C)2.3
D)4.3 × 10-3
E)0.54
At the start of the experiment, there are 0.200 mol of reactant (CH3NC)and 0 mol of product (CH3CN)in the reaction vessel. After 25 min of reaction, 0.108 mol of reactant (CH3NC)remain. The average rate of decomposition of methyl isonitrile, CH3NC, in this 25 min period is ________ mol/min.
A)3.7 × 10-3
B)0.092
C)2.3
D)4.3 × 10-3
E)0.54

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
42
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: ![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: How many moles of B are present at 10 s?</strong> A)0.011 B)0.220 C)0.110 D)0.014 E)1.4 × 10<sup>-3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_2626_a2ab_9b6fd935b612_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00.jpg)
How many moles of B are present at 10 s?
A)0.011
B)0.220
C)0.110
D)0.014
E)1.4 × 10-3
![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: How many moles of B are present at 10 s?</strong> A)0.011 B)0.220 C)0.110 D)0.014 E)1.4 × 10<sup>-3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_2626_a2ab_9b6fd935b612_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00.jpg)
How many moles of B are present at 10 s?
A)0.011
B)0.220
C)0.110
D)0.014
E)1.4 × 10-3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
43
The peroxydisulfate ion (S2O82-)reacts with the iodide ion in aqueous solution via the reaction:
S2O82- (aq) + 3I- → 2SO4 (aq)+ I3- (aq)
An aqueous solution containing 0.050 M of S2O82- ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below.![<strong>The peroxydisulfate ion (S<sub>2</sub>O<sub>8</sub><sup>2-</sup>)reacts with the iodide ion in aqueous solution via the reaction: S<sub>2</sub>O<sub>8</sub><sup>2-</sup> (aq) + 3I<sup>-</sup> → 2SO<sub>4</sub> (aq)+ I<sub>3</sub><sup>-</sup> (aq) An aqueous solution containing 0.050 M of S<sub>2</sub>O<sub>8</sub><sup>2-</sup> ion and 0.072 M of I<sup>-</sup> is prepared, and the progress of the reaction followed by measuring [I<sup>-</sup>]. The data obtained is given in the table below. The average rate of disappearance of I<sup>-</sup> between 400.0 s and 800.0 s is ________ M/s.</strong> A)2.8 × 10<sup>-5</sup> B)1.4 × 10<sup>-5</sup> C)5.8 × 10<sup>-5</sup> D)3.6 × 10<sup>4</sup> E)2.6 × 10<sup>-4</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_4d37_a2ab_fd7b3e9826f6_TB2701_00_TB2701_00_TB2701_00.jpg)
The average rate of disappearance of I- between 400.0 s and 800.0 s is ________ M/s.
A)2.8 × 10-5
B)1.4 × 10-5
C)5.8 × 10-5
D)3.6 × 104
E)2.6 × 10-4
S2O82- (aq) + 3I- → 2SO4 (aq)+ I3- (aq)
An aqueous solution containing 0.050 M of S2O82- ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below.
![<strong>The peroxydisulfate ion (S<sub>2</sub>O<sub>8</sub><sup>2-</sup>)reacts with the iodide ion in aqueous solution via the reaction: S<sub>2</sub>O<sub>8</sub><sup>2-</sup> (aq) + 3I<sup>-</sup> → 2SO<sub>4</sub> (aq)+ I<sub>3</sub><sup>-</sup> (aq) An aqueous solution containing 0.050 M of S<sub>2</sub>O<sub>8</sub><sup>2-</sup> ion and 0.072 M of I<sup>-</sup> is prepared, and the progress of the reaction followed by measuring [I<sup>-</sup>]. The data obtained is given in the table below. The average rate of disappearance of I<sup>-</sup> between 400.0 s and 800.0 s is ________ M/s.</strong> A)2.8 × 10<sup>-5</sup> B)1.4 × 10<sup>-5</sup> C)5.8 × 10<sup>-5</sup> D)3.6 × 10<sup>4</sup> E)2.6 × 10<sup>-4</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_4d37_a2ab_fd7b3e9826f6_TB2701_00_TB2701_00_TB2701_00.jpg)
The average rate of disappearance of I- between 400.0 s and 800.0 s is ________ M/s.
A)2.8 × 10-5
B)1.4 × 10-5
C)5.8 × 10-5
D)3.6 × 104
E)2.6 × 10-4

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
44
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: ![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: The average rate of appearance of B between 20 s and 30 s is ________ mol/s.</strong> A)+1.5 × 10<sup>-3</sup> B)+5.0 × 10<sup>-4</sup> C)-1.5 × 10<sup>-3</sup> D)+7.3 × 10<sup>-3</sup> E)-7.3 × 10<sup>-3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_2626_a2ab_9b6fd935b612_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00.jpg)
The average rate of appearance of B between 20 s and 30 s is ________ mol/s.
A)+1.5 × 10-3
B)+5.0 × 10-4
C)-1.5 × 10-3
D)+7.3 × 10-3
E)-7.3 × 10-3
![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: The average rate of appearance of B between 20 s and 30 s is ________ mol/s.</strong> A)+1.5 × 10<sup>-3</sup> B)+5.0 × 10<sup>-4</sup> C)-1.5 × 10<sup>-3</sup> D)+7.3 × 10<sup>-3</sup> E)-7.3 × 10<sup>-3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_2626_a2ab_9b6fd935b612_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00.jpg)
The average rate of appearance of B between 20 s and 30 s is ________ mol/s.
A)+1.5 × 10-3
B)+5.0 × 10-4
C)-1.5 × 10-3
D)+7.3 × 10-3
E)-7.3 × 10-3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
45
At elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2N2O5(g) → 4NO2 (g) + O2 (g)
When the rate of formation of NO2 is 5.5 × 10-4 M/s, the rate of decomposition of N2O5 is ________ M/s.
A)2.2 × 10-3
B)1.4 × 10-4
C)10.1 × 10-4
D)2.8 × 10-4
E)5.5 × 10-4
When the rate of formation of NO2 is 5.5 × 10-4 M/s, the rate of decomposition of N2O5 is ________ M/s.
A)2.2 × 10-3
B)1.4 × 10-4
C)10.1 × 10-4
D)2.8 × 10-4
E)5.5 × 10-4

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
46
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: ![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: The average rate of disappearance of A between 20 s and 40 s is ________ mol/s.</strong> A)8.5 × 10<sup>-4</sup> B)1.7 × 10<sup>-3</sup> C)590 D)7.1 × 10<sup>-3</sup> E)1.4 × 10<sup>-3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_2626_a2ab_9b6fd935b612_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00.jpg)
The average rate of disappearance of A between 20 s and 40 s is ________ mol/s.
A)8.5 × 10-4
B)1.7 × 10-3
C)590
D)7.1 × 10-3
E)1.4 × 10-3
![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: The average rate of disappearance of A between 20 s and 40 s is ________ mol/s.</strong> A)8.5 × 10<sup>-4</sup> B)1.7 × 10<sup>-3</sup> C)590 D)7.1 × 10<sup>-3</sup> E)1.4 × 10<sup>-3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_2626_a2ab_9b6fd935b612_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00.jpg)
The average rate of disappearance of A between 20 s and 40 s is ________ mol/s.
A)8.5 × 10-4
B)1.7 × 10-3
C)590
D)7.1 × 10-3
E)1.4 × 10-3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
47
The peroxydisulfate ion (S2O82-)reacts with the iodide ion in aqueous solution via the reaction:
S2O82- (aq) + 3I- → 2SO4 (aq)+ I3- (aq)
An aqueous solution containing 0.050 M of S2O82- ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below.![<strong>The peroxydisulfate ion (S<sub>2</sub>O<sub>8</sub><sup>2-</sup>)reacts with the iodide ion in aqueous solution via the reaction: S<sub>2</sub>O<sub>8</sub><sup>2-</sup> (aq) + 3I<sup>-</sup> → 2SO<sub>4</sub> (aq)+ I<sub>3</sub><sup>-</sup> (aq) An aqueous solution containing 0.050 M of S<sub>2</sub>O<sub>8</sub><sup>2-</sup> ion and 0.072 M of I<sup>-</sup> is prepared, and the progress of the reaction followed by measuring [I<sup>-</sup>]. The data obtained is given in the table below. The average rate of disappearance of I<sup>-</sup> between 1200.0 s and 1600.0 s is ________ M/s.</strong> A)1.8 × 10<sup>-5</sup> B)1.2 × 10<sup>-5</sup> C)2.0 × 10<sup>-5</sup> D)5.0 × 10<sup>4</sup> E)1.6 × 10<sup>-4</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_4d37_a2ab_fd7b3e9826f6_TB2701_00_TB2701_00_TB2701_00.jpg)
The average rate of disappearance of I- between 1200.0 s and 1600.0 s is ________ M/s.
A)1.8 × 10-5
B)1.2 × 10-5
C)2.0 × 10-5
D)5.0 × 104
E)1.6 × 10-4
S2O82- (aq) + 3I- → 2SO4 (aq)+ I3- (aq)
An aqueous solution containing 0.050 M of S2O82- ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below.
![<strong>The peroxydisulfate ion (S<sub>2</sub>O<sub>8</sub><sup>2-</sup>)reacts with the iodide ion in aqueous solution via the reaction: S<sub>2</sub>O<sub>8</sub><sup>2-</sup> (aq) + 3I<sup>-</sup> → 2SO<sub>4</sub> (aq)+ I<sub>3</sub><sup>-</sup> (aq) An aqueous solution containing 0.050 M of S<sub>2</sub>O<sub>8</sub><sup>2-</sup> ion and 0.072 M of I<sup>-</sup> is prepared, and the progress of the reaction followed by measuring [I<sup>-</sup>]. The data obtained is given in the table below. The average rate of disappearance of I<sup>-</sup> between 1200.0 s and 1600.0 s is ________ M/s.</strong> A)1.8 × 10<sup>-5</sup> B)1.2 × 10<sup>-5</sup> C)2.0 × 10<sup>-5</sup> D)5.0 × 10<sup>4</sup> E)1.6 × 10<sup>-4</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_4d37_a2ab_fd7b3e9826f6_TB2701_00_TB2701_00_TB2701_00.jpg)
The average rate of disappearance of I- between 1200.0 s and 1600.0 s is ________ M/s.
A)1.8 × 10-5
B)1.2 × 10-5
C)2.0 × 10-5
D)5.0 × 104
E)1.6 × 10-4

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
48
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: ![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: How many moles of B are present at 30 s?</strong> A)2.4 × 10<sup>-3</sup> B)0.15 C)0.073 D)1.7 × 10<sup>-3</sup> E)0.051](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_2626_a2ab_9b6fd935b612_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00.jpg)
How many moles of B are present at 30 s?
A)2.4 × 10-3
B)0.15
C)0.073
D)1.7 × 10-3
E)0.051
![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: How many moles of B are present at 30 s?</strong> A)2.4 × 10<sup>-3</sup> B)0.15 C)0.073 D)1.7 × 10<sup>-3</sup> E)0.051](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_2626_a2ab_9b6fd935b612_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00.jpg)
How many moles of B are present at 30 s?
A)2.4 × 10-3
B)0.15
C)0.073
D)1.7 × 10-3
E)0.051

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
49
A reaction was found to be second order in carbon monoxide concentration. The rate of the reaction ________ if the [CO] is doubled, with everything else kept the same.
A)doubles
B)remains unchanged
C)triples
D)increases by a factor of 4
E)is reduced by a factor of 2
A)doubles
B)remains unchanged
C)triples
D)increases by a factor of 4
E)is reduced by a factor of 2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
50
The concentration of S2O82- remaining at 1600 s is ________ M.
A)0.036
B)0.014
C)0.043
D)0.064
E)0.029
A)0.036
B)0.014
C)0.043
D)0.064
E)0.029

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the following reaction: A → 2C
The average rate of appearance of C is given by Δ[C]/Δt. Comparing the rate of appearance of C and the rate of disappearance of A, we get Δ[C]/Δt = ________ × (-Δ[A]/Δt).
A)+2
B)-1
C)+1
D)+1/2
E)-1/2
The average rate of appearance of C is given by Δ[C]/Δt. Comparing the rate of appearance of C and the rate of disappearance of A, we get Δ[C]/Δt = ________ × (-Δ[A]/Δt).
A)+2
B)-1
C)+1
D)+1/2
E)-1/2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
52
The active site of nitrogenase is a cofactor that contains two transition metals. These transition metals are ________.
A)Cr and Mg
B)Mn and V
C)Os and Ir
D)Fe and Zn
E)Fe and Mo
A)Cr and Mg
B)Mn and V
C)Os and Ir
D)Fe and Zn
E)Fe and Mo

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
53
The concentration of S2O82- remaining at 400 s is ________ M.
A)+0.015
B)+0.035
C)-0.007
D)+0.045
E)+0.057
A)+0.015
B)+0.035
C)-0.007
D)+0.045
E)+0.057

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
54
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: ![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: The average rate of disappearance of A between 10 s and 20 s is ________ mol/s.</strong> A)2.2 × 10<sup>-3</sup> B)1.1 × 10<sup>-3</sup> C)4.4 × 10<sup>-</sup><sup>3</sup> D)454 E)9.90 × 10<sup>-3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_2626_a2ab_9b6fd935b612_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00.jpg)
The average rate of disappearance of A between 10 s and 20 s is ________ mol/s.
A)2.2 × 10-3
B)1.1 × 10-3
C)4.4 × 10-3
D)454
E)9.90 × 10-3
![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: The average rate of disappearance of A between 10 s and 20 s is ________ mol/s.</strong> A)2.2 × 10<sup>-3</sup> B)1.1 × 10<sup>-3</sup> C)4.4 × 10<sup>-</sup><sup>3</sup> D)454 E)9.90 × 10<sup>-3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_2626_a2ab_9b6fd935b612_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00.jpg)
The average rate of disappearance of A between 10 s and 20 s is ________ mol/s.
A)2.2 × 10-3
B)1.1 × 10-3
C)4.4 × 10-3
D)454
E)9.90 × 10-3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
55
The concentration of S2O82- remaining at 800 s is ________ M.
A)0.046
B)0.076
C)4.00 × 10-3
D)0.015
E)0.041
A)0.046
B)0.076
C)4.00 × 10-3
D)0.015
E)0.041

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
56
Nitrogen fixation is a difficult process because ________.
A)there is so little nitrogen in the atmosphere
B)nitrogen exists in the atmosphere primarily as its oxides which are very unreactive
C)nitrogen is very unreactive, largely due to its triple bond
D)of the extreme toxicity of nitrogen
E)of the high polarity of nitrogen molecules preventing them from dissolving in biological fluids, such as those inside cells
A)there is so little nitrogen in the atmosphere
B)nitrogen exists in the atmosphere primarily as its oxides which are very unreactive
C)nitrogen is very unreactive, largely due to its triple bond
D)of the extreme toxicity of nitrogen
E)of the high polarity of nitrogen molecules preventing them from dissolving in biological fluids, such as those inside cells

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
57
The peroxydisulfate ion (S2O82-)reacts with the iodide ion in aqueous solution via the reaction:
S2O82- (aq) + 3I- → 2SO4 (aq)+ I3- (aq)
An aqueous solution containing 0.050 M of S2O82- ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below.![<strong>The peroxydisulfate ion (S<sub>2</sub>O<sub>8</sub><sup>2-</sup>)reacts with the iodide ion in aqueous solution via the reaction: S<sub>2</sub>O<sub>8</sub><sup>2-</sup> (aq) + 3I<sup>-</sup> → 2SO<sub>4</sub> (aq)+ I<sub>3</sub><sup>-</sup> (aq) An aqueous solution containing 0.050 M of S<sub>2</sub>O<sub>8</sub><sup>2-</sup> ion and 0.072 M of I<sup>-</sup> is prepared, and the progress of the reaction followed by measuring [I<sup>-</sup>]. The data obtained is given in the table below. The average rate of disappearance of I<sup>-</sup> in the initial 400.0 s is ________ M/s.</strong> A)6.00 B)3.8 × 10<sup>-5</sup> C)1.4 × 10<sup>-4</sup> D)2.7 × 10<sup>4</sup> E)3.2 × 10<sup>-4</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_4d37_a2ab_fd7b3e9826f6_TB2701_00_TB2701_00_TB2701_00.jpg)
The average rate of disappearance of I- in the initial 400.0 s is ________ M/s.
A)6.00
B)3.8 × 10-5
C)1.4 × 10-4
D)2.7 × 104
E)3.2 × 10-4
S2O82- (aq) + 3I- → 2SO4 (aq)+ I3- (aq)
An aqueous solution containing 0.050 M of S2O82- ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below.
![<strong>The peroxydisulfate ion (S<sub>2</sub>O<sub>8</sub><sup>2-</sup>)reacts with the iodide ion in aqueous solution via the reaction: S<sub>2</sub>O<sub>8</sub><sup>2-</sup> (aq) + 3I<sup>-</sup> → 2SO<sub>4</sub> (aq)+ I<sub>3</sub><sup>-</sup> (aq) An aqueous solution containing 0.050 M of S<sub>2</sub>O<sub>8</sub><sup>2-</sup> ion and 0.072 M of I<sup>-</sup> is prepared, and the progress of the reaction followed by measuring [I<sup>-</sup>]. The data obtained is given in the table below. The average rate of disappearance of I<sup>-</sup> in the initial 400.0 s is ________ M/s.</strong> A)6.00 B)3.8 × 10<sup>-5</sup> C)1.4 × 10<sup>-4</sup> D)2.7 × 10<sup>4</sup> E)3.2 × 10<sup>-4</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_4d37_a2ab_fd7b3e9826f6_TB2701_00_TB2701_00_TB2701_00.jpg)
The average rate of disappearance of I- in the initial 400.0 s is ________ M/s.
A)6.00
B)3.8 × 10-5
C)1.4 × 10-4
D)2.7 × 104
E)3.2 × 10-4

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
58
Consider the following reaction: 3A → 2B
The average rate of appearance of B is given by Δ[B]/Δt. Comparing the rate of appearance of B and the rate of disappearance of A, we get Δ[B]/Δt = ________ × (-Δ[A]/Δt).
A)-2/3
B)+2/3
C)-3/2
D)+1
E)+3/2
The average rate of appearance of B is given by Δ[B]/Δt. Comparing the rate of appearance of B and the rate of disappearance of A, we get Δ[B]/Δt = ________ × (-Δ[A]/Δt).
A)-2/3
B)+2/3
C)-3/2
D)+1
E)+3/2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
59
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: ![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: The average rate disappearance of A between 20 s and 30 s is ________ mol/s.</strong> A)5.0 × 10<sup>-4</sup> B)1.6 × 10<sup>-2</sup> C)1.5 × 10<sup>-3</sup> D)670 E)0.15](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_2626_a2ab_9b6fd935b612_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00.jpg)
The average rate disappearance of A between 20 s and 30 s is ________ mol/s.
A)5.0 × 10-4
B)1.6 × 10-2
C)1.5 × 10-3
D)670
E)0.15
![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g)→B(g). The following data are obtained for [A] as the reaction proceeds: The average rate disappearance of A between 20 s and 30 s is ________ mol/s.</strong> A)5.0 × 10<sup>-4</sup> B)1.6 × 10<sup>-2</sup> C)1.5 × 10<sup>-3</sup> D)670 E)0.15](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_2626_a2ab_9b6fd935b612_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00.jpg)
The average rate disappearance of A between 20 s and 30 s is ________ mol/s.
A)5.0 × 10-4
B)1.6 × 10-2
C)1.5 × 10-3
D)670
E)0.15

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
60
Which substance in the reaction below either appears or disappears the fastest? 4NH3 + 7O2 → 4NO2 + 6H2O
A)NH3
B)O2
C)NO2
D)H2O
E)The rates of appearance/disappearance are the same for all of these.
A)NH3
B)O2
C)NO2
D)H2O
E)The rates of appearance/disappearance are the same for all of these.

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
61
Of the following, only ________ is a valid unit for reaction rate.
A)M/s
B)mmol/mL
C)mol/g
D)g/L
E)atm/g
A)M/s
B)mmol/mL
C)mol/g
D)g/L
E)atm/g

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
62
The overall order of a reaction is 2. The units of the rate constant for the reaction are ________.
A)M/s
B)M-1s-1
C)1/s
D)1/M
E)s/M2
A)M/s
B)M-1s-1
C)1/s
D)1/M
E)s/M2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
63
The data in the table below were obtained for the reaction:
A + B → P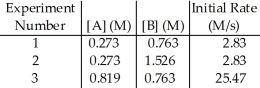
The order of the reaction in A is ________.
A)1
B)2
C)3
D)4
E)0
A + B → P

The order of the reaction in A is ________.
A)1
B)2
C)3
D)4
E)0

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
64
A reaction was found to be zero order in A. Increasing the concentration of A by a factor of 3 will cause the reaction rate to ________.
A)remain constant
B)increase by a factor of 27
C)increase by a factor of 9
D)triple
E)decrease by a factor of the cube root of 3
A)remain constant
B)increase by a factor of 27
C)increase by a factor of 9
D)triple
E)decrease by a factor of the cube root of 3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
65
The overall order of a reaction is 1. The units of the rate constant for the reaction are ________.
A)M/s
B)M-1s-1
C)1/s
D)1/M
E)s/M2
A)M/s
B)M-1s-1
C)1/s
D)1/M
E)s/M2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
66
The data in the table below were obtained for the reaction:
A + B → P
The overall order of the reaction is ________.
A)1
B)2
C)3
D)4
E)0
A + B → P

The overall order of the reaction is ________.
A)1
B)2
C)3
D)4
E)0

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
67
A second-order reaction has a half-life of 12 s when the initial concentration of reactant is 0.98 M. The rate constant for this reaction is ________ M-1s-1.
A)12
B)2.0 × 10-2
C)8.5 × 10-2
D)4.3 × 10-2
E)4.3
A)12
B)2.0 × 10-2
C)8.5 × 10-2
D)4.3 × 10-2
E)4.3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
68
For a first-order reaction, a plot of ________ versus ________ is linear.
A)ln [A]t,![<strong>For a first-order reaction, a plot of ________ versus ________ is linear.</strong> A)ln [A]<sub>t</sub>, B)ln [A]<sub>t</sub>, t C) , t D)[A]<sub>t</sub>, t E)t,](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_e97a_a2ab_df98b2796de5_TB2701_11.jpg)
B)ln [A]t, t
C)![<strong>For a first-order reaction, a plot of ________ versus ________ is linear.</strong> A)ln [A]<sub>t</sub>, B)ln [A]<sub>t</sub>, t C) , t D)[A]<sub>t</sub>, t E)t,](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_e97b_a2ab_1bdf2613abfc_TB2701_11.jpg) , t
, t
D)[A]t, t
E)t,![<strong>For a first-order reaction, a plot of ________ versus ________ is linear.</strong> A)ln [A]<sub>t</sub>, B)ln [A]<sub>t</sub>, t C) , t D)[A]<sub>t</sub>, t E)t,](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f64_108c_a2ab_9bcdff11abad_TB2701_11.jpg)
A)ln [A]t,
![<strong>For a first-order reaction, a plot of ________ versus ________ is linear.</strong> A)ln [A]<sub>t</sub>, B)ln [A]<sub>t</sub>, t C) , t D)[A]<sub>t</sub>, t E)t,](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_e97a_a2ab_df98b2796de5_TB2701_11.jpg)
B)ln [A]t, t
C)
![<strong>For a first-order reaction, a plot of ________ versus ________ is linear.</strong> A)ln [A]<sub>t</sub>, B)ln [A]<sub>t</sub>, t C) , t D)[A]<sub>t</sub>, t E)t,](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_e97b_a2ab_1bdf2613abfc_TB2701_11.jpg) , t
, t D)[A]t, t
E)t,
![<strong>For a first-order reaction, a plot of ________ versus ________ is linear.</strong> A)ln [A]<sub>t</sub>, B)ln [A]<sub>t</sub>, t C) , t D)[A]<sub>t</sub>, t E)t,](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f64_108c_a2ab_9bcdff11abad_TB2701_11.jpg)

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
69
A reaction was found to be third order in A. Increasing the concentration of A by a factor of 3 will cause the reaction rate to ________.
A)remain constant
B)increase by a factor of 27
C)increase by a factor of 9
D)triple
E)decrease by a factor of the cube root of 3
A)remain constant
B)increase by a factor of 27
C)increase by a factor of 9
D)triple
E)decrease by a factor of the cube root of 3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
70
The graph shown below depicts the relationship between concentration and time for the following chemical reaction. ![<strong>The graph shown below depicts the relationship between concentration and time for the following chemical reaction. The slope of this line is equal to ________.</strong> A)k B)-1/k C)ln [A]<sub>o</sub> D)-k E)1/k](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f64_108d_a2ab_77028f1f7b2a_TB2701_00.jpg) The slope of this line is equal to ________.
The slope of this line is equal to ________.
A)k
B)-1/k
C)ln [A]o
D)-k
E)1/k
![<strong>The graph shown below depicts the relationship between concentration and time for the following chemical reaction. The slope of this line is equal to ________.</strong> A)k B)-1/k C)ln [A]<sub>o</sub> D)-k E)1/k](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f64_108d_a2ab_77028f1f7b2a_TB2701_00.jpg) The slope of this line is equal to ________.
The slope of this line is equal to ________.A)k
B)-1/k
C)ln [A]o
D)-k
E)1/k

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
71
If the rate law for the reaction 2A + 3B → products
Is first order in A and second order in B, then the rate law is rate = ________.
A)k[A][B]
B)k[A]2[B]3
C)k[A][B]2
D)k[A]2[B]
E)k[A]2[B]2
Is first order in A and second order in B, then the rate law is rate = ________.
A)k[A][B]
B)k[A]2[B]3
C)k[A][B]2
D)k[A]2[B]
E)k[A]2[B]2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
72
The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13 M, it takes ________ min for it to decrease to 0.085 M.
A)12
B)10.
C)8.0
D)11
E)7.0
A)12
B)10.
C)8.0
D)11
E)7.0

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
73
A second-order reaction has a half-life of 18 s when the initial concentration of reactant is 0.71 M. The rate constant for this reaction is ________ M-1s-1.
A)7.8 × 10-2
B)3.8 × 10-2
C)2.0 × 10-2
D)1.3
E)18
A)7.8 × 10-2
B)3.8 × 10-2
C)2.0 × 10-2
D)1.3
E)18

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
74
The reaction below is first order in [H2O2]: 2H2O2 (l) → 2H2O (l) + O2 (g)
A solution originally at 0.600 M H2O2 is found to be 0.075 M after 54 min. The half-life for this reaction is ________ min.
A)6.8
B)18
C)14
D)28
E)54
A solution originally at 0.600 M H2O2 is found to be 0.075 M after 54 min. The half-life for this reaction is ________ min.
A)6.8
B)18
C)14
D)28
E)54

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
75
If the rate law for the reaction 2A + 3B → products
Is second order in A and first order in B, then the rate law is rate = ________.
A)k[A][B]
B)k[A]2[B]3
C)k[A][B]2
D)k[A]2[B]
E)k[A]2[B]2
Is second order in A and first order in B, then the rate law is rate = ________.
A)k[A][B]
B)k[A]2[B]3
C)k[A][B]2
D)k[A]2[B]
E)k[A]2[B]2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
76
The following reaction occurs in aqueous solution: NH4+ (aq) + NO2- → N2 (g) + 2H2O (l)
The data below is obtained at 25 °C.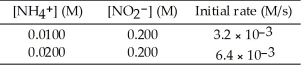 The order of the reaction in NH4+ is ________.
The order of the reaction in NH4+ is ________.
A)-2
B)-1
C)+2
D)+1
E)0
The data below is obtained at 25 °C.
 The order of the reaction in NH4+ is ________.
The order of the reaction in NH4+ is ________.A)-2
B)-1
C)+2
D)+1
E)0

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
77
The data in the table below were obtained for the reaction:
A + B → P
The order of the reaction in B is ________.
A)1
B)2
C)3
D)4
E)0
A + B → P

The order of the reaction in B is ________.
A)1
B)2
C)3
D)4
E)0

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
78
The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.085 M, it takes ________ min for it to decrease to 0.055 M.
A)8.2
B)11
C)3.6
D)0.048
E)8.4
A)8.2
B)11
C)3.6
D)0.048
E)8.4

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
79
The kinetics of the reaction below were studied and it was determined that the reaction rate did not change when the concentration of B was tripled. The reaction is ________ order in B. A + B → P
A)zero
B)first
C)second
D)third
E)one-half
A)zero
B)first
C)second
D)third
E)one-half

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
80
The kinetics of the reaction below were studied and it was determined that the reaction rate increased by a factor of 9 when the concentration of B was tripled. The reaction is ________ order in B. A + B → P
A)zero
B)first
C)second
D)third
E)one-half
A)zero
B)first
C)second
D)third
E)one-half

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck



