Deck 10: Elimination Reactions of Alkyl Halides Competition Between Substitution and Elimination
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/94
Play
Full screen (f)
Deck 10: Elimination Reactions of Alkyl Halides Competition Between Substitution and Elimination
1
Give the major product for the following E2 reaction. 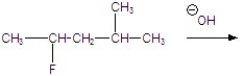
A)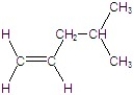
B)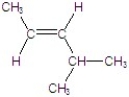
C)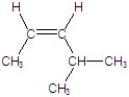
D)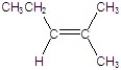
E)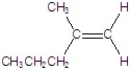

A)

B)

C)

D)

E)


2
Which of the alkyl chlorides listed below undergoes dehydrohalogenation in the presence of a strong base to give 2-pentene as the only alkene product?
A)1-chloropentane
B)2-chloropentane
C)3-chloropentane
D)1-chloro-2-methylbutane
E)1-chloro-3-methylbutane
A)1-chloropentane
B)2-chloropentane
C)3-chloropentane
D)1-chloro-2-methylbutane
E)1-chloro-3-methylbutane
3-chloropentane
3
How many distinct alkene products are possible when the alkyl iodide below undergoes E2 elimination? 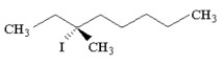
A)1
B)2
C)3
D)4
E)5

A)1
B)2
C)3
D)4
E)5
5
4
Which of the following bases gives the highest anti-Zaitsev product in E2 reactions when reacted with 2-bromo-2, 3-dimethylbutane?
A)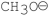
B)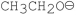
C)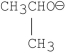
D)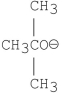
E)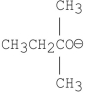
A)
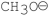
B)
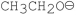
C)
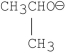
D)

E)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
5
Draw the alkene product which results when 1-bromopentane is heated in acetone containing NaOH. Give a detailed, step-by-step mechanism for the production of this compound.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
6
When 2-bromo-3-methyl-1-phenylbutane is treated with sodium methoxide, why is the major product 3-methyl-1-phenyl-1-butene?
A)SN2 predominates over E2.
B)E1 predominates over E2.
C)The bulkiness of the methoxide results in the less substituted alkene.
D)The newly formed double bond in this compound is conjugated with the phenyl ring.
E)The less substituted alkene is always more stable than the more substituted alkene.
A)SN2 predominates over E2.
B)E1 predominates over E2.
C)The bulkiness of the methoxide results in the less substituted alkene.
D)The newly formed double bond in this compound is conjugated with the phenyl ring.
E)The less substituted alkene is always more stable than the more substituted alkene.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following halides is most reactive in an E2 reaction with sodium methoxide?
A)(CH3)3CCH2I
B)(CH3)2CHCHICH3
C)(CH3)2CHCH2Br
D)(CH3)2CHCH2Cl
E)(CH3)2CHCH2CH2Cl
A)(CH3)3CCH2I
B)(CH3)2CHCHICH3
C)(CH3)2CHCH2Br
D)(CH3)2CHCH2Cl
E)(CH3)2CHCH2CH2Cl

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following compounds undergoes E2 reactions with the fastest rate?
A)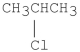
B)CH3CH2CH2Cl
C)CH3CH2CH2I
D)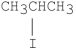
E)CH3CH2CH2Br
A)
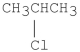
B)CH3CH2CH2Cl
C)CH3CH2CH2I
D)
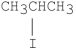
E)CH3CH2CH2Br

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
9
What is the major organic product of the following reaction? 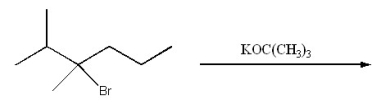
A)2,3-dimethyl-1-hexene
B)2,3-dimethyl-2-hexene
C)2-isopropyl-1-pentene
D)(Z)-2,3-dimethyl-3-hexene
E)(E)-2,3-dimethyl-3-hexene
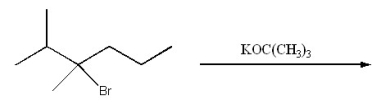
A)2,3-dimethyl-1-hexene
B)2,3-dimethyl-2-hexene
C)2-isopropyl-1-pentene
D)(Z)-2,3-dimethyl-3-hexene
E)(E)-2,3-dimethyl-3-hexene

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
10
What is the most reactive alkyl halide for elimination reactions?
A)1-chlorobutane
B)1-fluorobutane
C)1-iodobutane
D)1-bromobutane
A)1-chlorobutane
B)1-fluorobutane
C)1-iodobutane
D)1-bromobutane

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
11
Give the major product for the following E2 reaction. 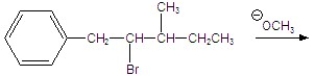
A)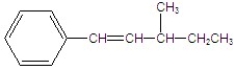
B)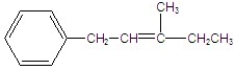
C)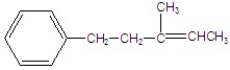
D)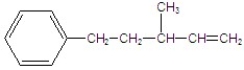
E)none of the above

A)
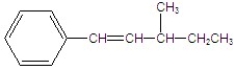
B)
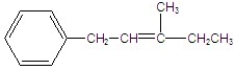
C)
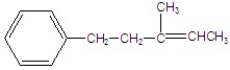
D)
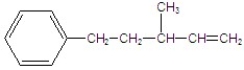
E)none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
12
What is the major organic product of the following reaction? 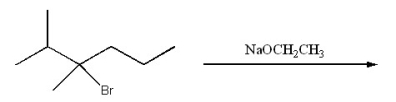
A)2,3-dimethyl-1-hexene
B)2,3-dimethyl-2-hexene
C)2-isopropyl-1-pentene
D)(Z)-2,3-dimethyl-3-hexene
E)(E)-2,3-dimethyl-3-hexene
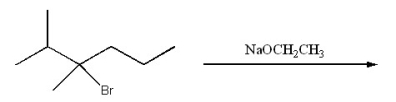
A)2,3-dimethyl-1-hexene
B)2,3-dimethyl-2-hexene
C)2-isopropyl-1-pentene
D)(Z)-2,3-dimethyl-3-hexene
E)(E)-2,3-dimethyl-3-hexene

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following halides is least reactive in an E2 reaction with sodium methoxide?
A)(CH3)3CCH2I
B)(CH3)2CHCHICH3
C)(CH3)2CHCH2Br
D)(CH3)2CHCH2Cl
E)(CH3)2CHCH2CH2Cl
A)(CH3)3CCH2I
B)(CH3)2CHCHICH3
C)(CH3)2CHCH2Br
D)(CH3)2CHCH2Cl
E)(CH3)2CHCH2CH2Cl

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following are the elimination products of the reaction shown below? CH3CH2Br + -OH → ?
A)CH3CH2Br+H + O-
B)HOCH2CH2Br
C)CH3CH2OH + Br-
D)CH2 CH2 + Br- + H2O
CH2 + Br- + H2O
E)CH2 CHBr + H2O
CHBr + H2O
A)CH3CH2Br+H + O-
B)HOCH2CH2Br
C)CH3CH2OH + Br-
D)CH2
 CH2 + Br- + H2O
CH2 + Br- + H2OE)CH2
 CHBr + H2O
CHBr + H2O
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
15
Give the mechanism of the reaction shown below. 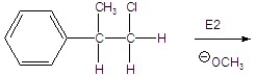


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
16
Which alkyl halide reacts the fastest in an E2 reaction?
A)2-chloro-2-methylbutane
B)1-chlorobutane
C)1-chloro-2-methylbutane
D)2-chlorobutane
E)chloromethane
A)2-chloro-2-methylbutane
B)1-chlorobutane
C)1-chloro-2-methylbutane
D)2-chlorobutane
E)chloromethane

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
17
Give the mechanism of the reaction shown below. 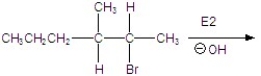


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
18
What is the major product of the following E2 reaction? 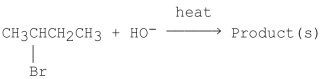
A)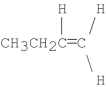
B)CH3CH2CH CH2
CH2
C)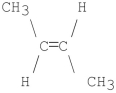
D)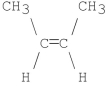
E)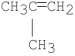

A)
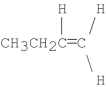
B)CH3CH2CH
 CH2
CH2C)

D)

E)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
19
Give the major product for the E2 reaction. 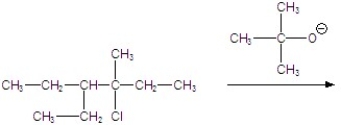
A)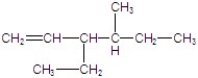
B)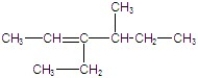
C)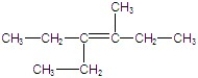
D)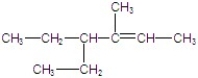
E)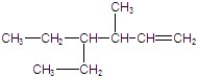

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements is true concerning the E2 reactions of alkyl fluorides?
A)Alkyl fluorides react more readily in E2 reactions than do alkyl iodides.
B)The transition state of this E2 reaction resembles a carbanion rather than an alkene.
C)The C-F bond is the weakest carbon-halogen bond so SN2 will always predominate over E2 in the reactions of alkyl fluorides.
D)Alkyl fluorides react to form the most stable carbocation intermediate.
E)Alkyl fluorides cannot adopt the proper stereochemical alignment for an E2 reaction to occur.
A)Alkyl fluorides react more readily in E2 reactions than do alkyl iodides.
B)The transition state of this E2 reaction resembles a carbanion rather than an alkene.
C)The C-F bond is the weakest carbon-halogen bond so SN2 will always predominate over E2 in the reactions of alkyl fluorides.
D)Alkyl fluorides react to form the most stable carbocation intermediate.
E)Alkyl fluorides cannot adopt the proper stereochemical alignment for an E2 reaction to occur.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
21
When 1-iodo-1-methylcyclohexane is treated with NaOCH2CH3, the more highly substituted alkene product predominates. When KOC(CH3)3 is used instead, the less highly substituted alkene product predominates. Offer an explanation.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following statements correctly describe(s)E1 reactions of alkyl halides (RX)? I. Rate = k[base]
II. Rate = k[base][RX]
III. Rate = k[RX]
IV. The reactions occur in two distinct steps.
V. Rearrangements are sometimes seen.
A)II and IV
B)III and V
C)I, IV, and V
D)I only
E)III, IV, and V
II. Rate = k[base][RX]
III. Rate = k[RX]
IV. The reactions occur in two distinct steps.
V. Rearrangements are sometimes seen.
A)II and IV
B)III and V
C)I, IV, and V
D)I only
E)III, IV, and V

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following alkyl halides undergoes E1 reactions with the fastest rate?
A)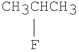
B)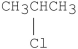
C)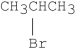
D)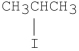
E)CH3I
A)
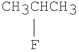
B)
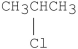
C)
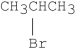
D)
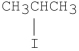
E)CH3I

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
24
Provide the major organic product of the following reaction. 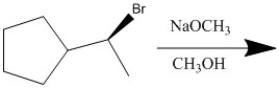


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
25
Provide the major organic product of the following reaction. 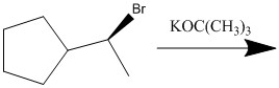


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following correctly reflects relative stabilities of carbocations?
A)3° allylic > 2° > 1° benzylic
B)methyl > 2° benzylic > 3°
C)3° benzylic > vinyl > 1°
D)2° allylic > 2° > vinyl
E)1° benzylic > 3° > 3° allylic
A)3° allylic > 2° > 1° benzylic
B)methyl > 2° benzylic > 3°
C)3° benzylic > vinyl > 1°
D)2° allylic > 2° > vinyl
E)1° benzylic > 3° > 3° allylic

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
27
When 1-bromo-2,2-dimethylcyclopentane is heated in ethanol, one of the products which results is shown below. Provide a detailed, stepwise mechanism for the production of this compound, and give the name of the mechanism by which it is produced. 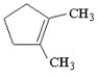


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
28
Provide the major organic product of the following reaction. 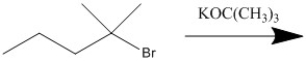


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
29
Consider the following experimental data for the rate of the reaction given below: 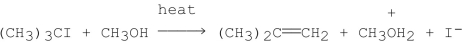
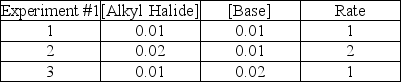 What is the mechanism for the reaction?
What is the mechanism for the reaction?
A)first order, SN1
B)first order, SN2
C)first order, E1
D)first order, E2
E)none of the above
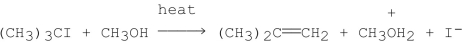
 What is the mechanism for the reaction?
What is the mechanism for the reaction?A)first order, SN1
B)first order, SN2
C)first order, E1
D)first order, E2
E)none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
30
Provide the major organic product of the following reaction. 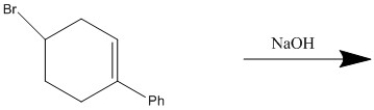


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following alkyl halides forms the most stable carbocation when it undergoes an E1 reaction? 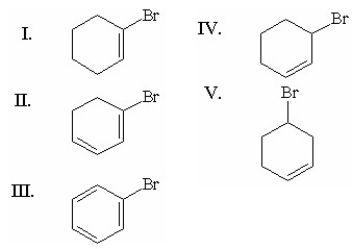
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
32
Provide the structure of the major organic product which results when 2-bromo-2-methylbutane is treated with sodium ethoxide.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
33
Supply the missing alkyl halide reactant in the elimination reactions shown below. 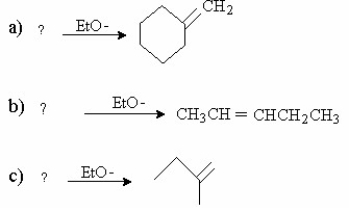


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
34
Draw all likely alkene products in the following reaction and circle the product you expect to predominate. 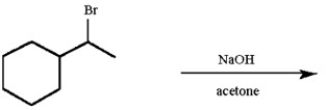
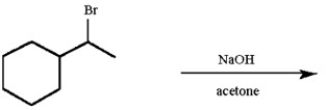

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
35
List the following compounds in order of increasing reactivity in an E1 elimination.
CH3CH2CHBrCH3, CH3CH2CH2CH2Br, (CH3)3CBr
CH3CH2CHBrCH3, CH3CH2CH2CH2Br, (CH3)3CBr

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
36
Provide the structure of the major organic product of the following reaction. 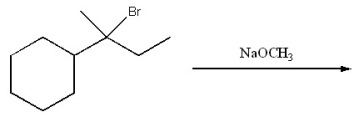


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
37
Draw all likely alkene products in the following reaction and circle the product you expect to predominate. 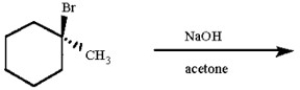


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
38
Provide the structure of the major organic product of the following reaction. 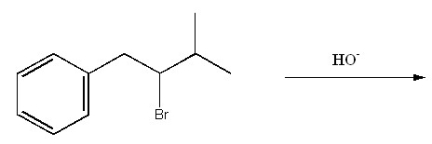


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
39
Draw all likely alkene products in the following reaction and circle the product you expect to predominate. 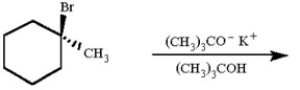


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
40
Provide the major organic product of the following reaction. 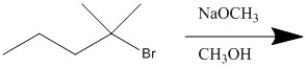


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the compounds shown below is/are the product(s)of this reaction? 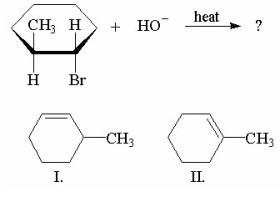
A)I only
B)II only
C)I and II are of equal yield.
D)I is major, II is minor.
E)I is minor, II is major.

A)I only
B)II only
C)I and II are of equal yield.
D)I is major, II is minor.
E)I is minor, II is major.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
42
Give the major product for the following E1 reaction. 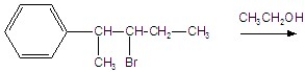
A)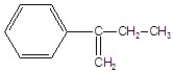
B)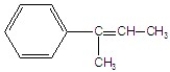
C)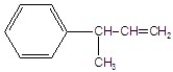
D)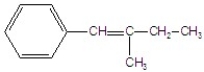
E)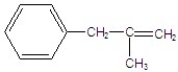

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
43
Dehydrohalogenation of 2-bromobutane in the presence of a strong base proceeds via which of the following mechanistic pathways?
A)SN1
B)SN2
C)E1
D)E2
E)none of the above
A)SN1
B)SN2
C)E1
D)E2
E)none of the above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
44
Provide the structure of the major organic product in the following reaction. 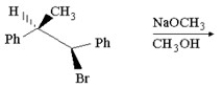


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
45
Give the major product for the following E2 reaction. 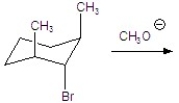
A)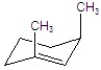
B)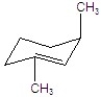
C)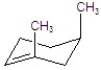
D)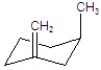
E)no reaction

A)

B)

C)

D)

E)no reaction

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
46
Why is the E1 not a likely mechanism when 1-chloropentane is heated in ethanol?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
47
Which diastereomer of 1-bromo-4-t-butylcyclohexane, the cis or the trans, undergoes elimination more rapidly when treated with sodium ethoxide? Explain your answer.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is least likely to be found in the product mixture which results when 2-iodopentane reacts with sodium ethoxide in ethanol?
A)1-ethoxypentane
B)2-ethoxypentane
C)(Z)-2-pentene
D)(E)-2-pentene
E)1-pentene
A)1-ethoxypentane
B)2-ethoxypentane
C)(Z)-2-pentene
D)(E)-2-pentene
E)1-pentene

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
49
Why is the alkyl halide below not capable of undergoing an E2 reaction upon treatment with sodium ethoxide? 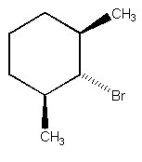
A)Br- is too poor a leaving group.
B)The substrate is too hindered.
C)Too much angle strain would be present in the alkene product.
D)Sodium ethoxide is a poor base to use in E2 reactions.
E)The C-H and C-Br bonds which need to break cannot achieve an anti-periplanar orientation.

A)Br- is too poor a leaving group.
B)The substrate is too hindered.
C)Too much angle strain would be present in the alkene product.
D)Sodium ethoxide is a poor base to use in E2 reactions.
E)The C-H and C-Br bonds which need to break cannot achieve an anti-periplanar orientation.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
50
Provide the structure of the major organic product which results in the following reaction. 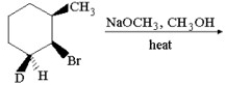


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
51
What is the major product which results when (2R,3S)-2-chloro-3-phenylbutane is treated with sodium methoxide in methanol?
A)(E)-2-phenyl-2-butene
B)(Z)-2-phenyl-2-butene
C)(S)-3-phenyl-1-butene
D)(R)-3-phenyl-1-butene
E)(R)-2-methoxy-2-phenylbutane
A)(E)-2-phenyl-2-butene
B)(Z)-2-phenyl-2-butene
C)(S)-3-phenyl-1-butene
D)(R)-3-phenyl-1-butene
E)(R)-2-methoxy-2-phenylbutane

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
52
Identify the alkyl halide that reacts the fastest in a E1 reaction.
A)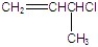
B)
C)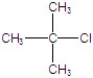
D)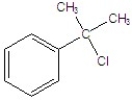
E)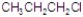
A)
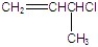
B)

C)

D)

E)
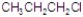

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
53
A primary kinetic isotope effect could most likely be observed in which of the following mechanisms?
A)SN1 and SN2
B)SN2
C)E1 and SN1
D)E2
E)E1
A)SN1 and SN2
B)SN2
C)E1 and SN1
D)E2
E)E1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
54
Give the major product for the following E1 reaction. 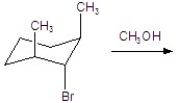
A)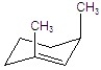
B)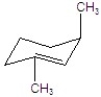
C)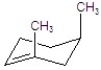
D)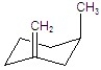
E)no reaction

A)

B)

C)

D)

E)no reaction

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
55
Give the mechanism of the reaction shown below. 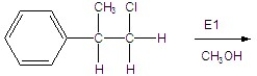


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
56
Give the mechanism of the reaction shown below. 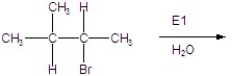


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
57
Provide the structure of the major elimination product which results when the alkyl bromide below is heated in ethanol. 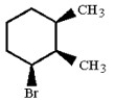


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
58
Provide the structure of the major organic product which results in the following reaction. 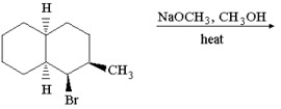


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following compounds is/are the products of the following reaction? 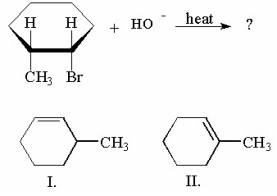
A)I only
B)II only
C)I and II are of equal yield.
D)I is major, II is minor.
E)I is minor, II is major.

A)I only
B)II only
C)I and II are of equal yield.
D)I is major, II is minor.
E)I is minor, II is major.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
60
Provide the structure of the major organic product which results in the following reaction. 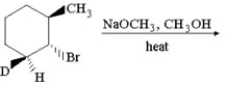


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
61
Give the major product for the following reaction. 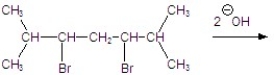
A)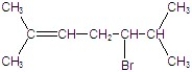
B)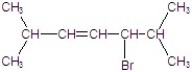
C)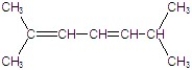
D)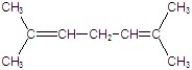
E)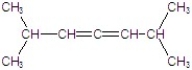

A)

B)

C)
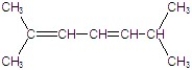
D)
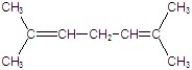
E)
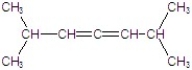

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
62
Provide the structure of the major organic product of the following reaction. 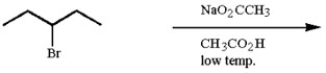
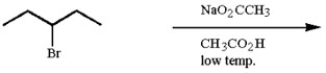

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
63
What is the major product of the following reaction? 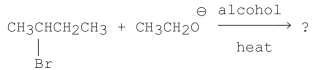
A)CH2 CHCH2CH3
CHCH2CH3
B)CH3CH CHCH3
CHCH3
C)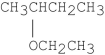
D)CH3CH2CH2CH2OCH2CH3
E)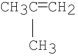
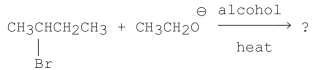
A)CH2
 CHCH2CH3
CHCH2CH3B)CH3CH
 CHCH3
CHCH3C)
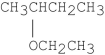
D)CH3CH2CH2CH2OCH2CH3
E)
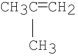

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
64
What mechanism predominates in the reaction below? 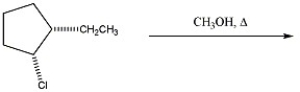
A)SN2
B)SN1 without rearrangement
C)SN1 with rearrangement
D)E2
E)E1

A)SN2
B)SN1 without rearrangement
C)SN1 with rearrangement
D)E2
E)E1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
65
What mechanism predominates in the reaction below? 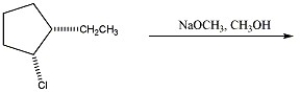
A)SN2
B)SN1 without rearrangement
C)SN1 with rearrangement
D)E2
E)E1

A)SN2
B)SN1 without rearrangement
C)SN1 with rearrangement
D)E2
E)E1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
66
What is/are the product(s)of the following reaction? 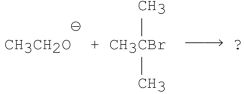
A)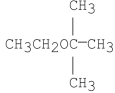
B)CH2 CH2
CH2
C)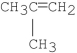
D)A and B
E)A and C
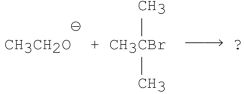
A)

B)CH2
 CH2
CH2C)
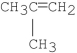
D)A and B
E)A and C

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
67
What is/are the product(s)of the following reaction? 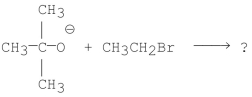
A)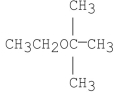
B)CH2 CH2
CH2
C)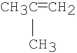
D)A and B
E)A and C
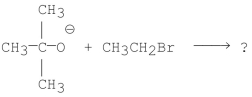
A)

B)CH2
 CH2
CH2C)
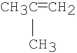
D)A and B
E)A and C

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
68
Provide the major organic product(s)in the reaction below. 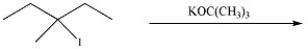
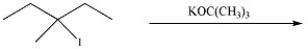

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
69
Can primary alkyl halides react by SN2, SN1, E2, and E1 mechanisms? Are any of these mechanisms prohibited? What conditions favor a particular mechanism?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
70
Predict the two most likely mechanisms which occur when 2-iodohexane is heated in ethanol.
A)SN2 and SN1
B)E1 and E2
C)SN2 and E2
D)E1 and SN1
E)E2 and SN1
A)SN2 and SN1
B)E1 and E2
C)SN2 and E2
D)E1 and SN1
E)E2 and SN1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
71
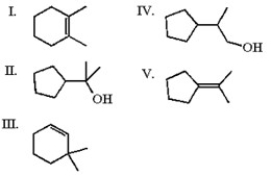
Which of the following is least likely to be found in the product mixture which results when the alkyl iodide below is heated in water? 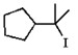
A)I
B)II
C)III
D)IV
E)V


A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
72
What reaction mechanism predominates when 1-bromo-1-propylcyclopentane is treated with sodium methoxide in methanol?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
73
Give the best answer for the reaction. 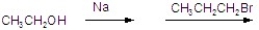
A)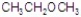
B)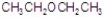
C)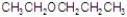
D)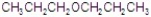
E)
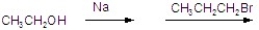
A)
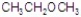
B)
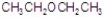
C)
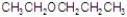
D)
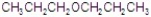
E)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
74
What is the major product of the following reaction? 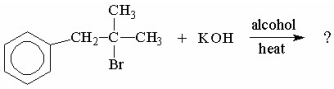
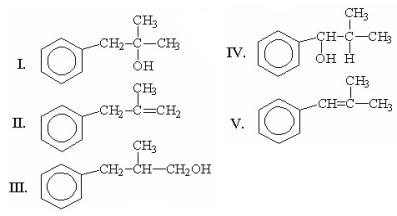
A)I
B)II
C)III
D)IV
E)V
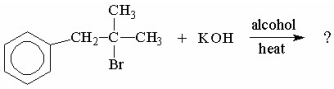

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
75
What reaction mechanism predominates when 1-bromo-1-propylcyclopentane is heated in ethanol?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
76
Which base, ammonia (NH3)or triethylamine [(CH3CH2)3N], would be a better choice for use in converting 1-chlorohexane to 1-hexene? Explain briefly.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
77
What is the major product of the following reaction? 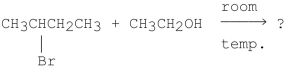
A)CH2 CHCH2CH3
CHCH2CH3
B)CH3CH CHCH3
CHCH3
C)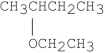
D)CH3CH2CH2CH2OCH2CH3
E)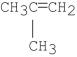
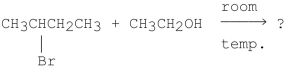
A)CH2
 CHCH2CH3
CHCH2CH3B)CH3CH
 CHCH3
CHCH3C)
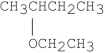
D)CH3CH2CH2CH2OCH2CH3
E)
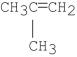

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
78
Give the major product(s)for the following reaction. 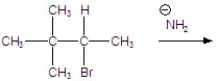
A)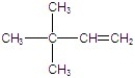
B)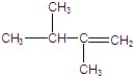
C)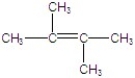
D)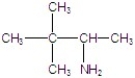
E)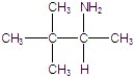

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
79
What mechanism predominates in the reaction below? 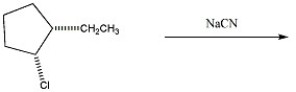
A)SN2
B)SN1 without rearrangement
C)SN1 with rearrangement
D)E2
E)E1

A)SN2
B)SN1 without rearrangement
C)SN1 with rearrangement
D)E2
E)E1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
80
Predict the two most likely mechanisms for the reaction of 2-iodohexane with sodium ethoxide.
A)SN2 and SN1
B)E1 and E2
C)SN2 and E2
D)E1 and SN1
E)E2 and SN1
A)SN2 and SN1
B)E1 and E2
C)SN2 and E2
D)E1 and SN1
E)E2 and SN1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck



