Deck 14: Chemical Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/171
Play
Full screen (f)
Deck 14: Chemical Equilibrium
1
If Kc is the equilibrium constant for a forward reaction what is Kc' for the reverse reaction?
A)- Kc
B)Kc
C)(Kc)-1
D)none of these
A)- Kc
B)Kc
C)(Kc)-1
D)none of these
(Kc)-1
2
Write the equilibrium equation for the reverse reaction: 2 CH4(g)+ 3 O2(g)⇌ 2 CO(g)+ 4 H2O(g)
A)Kp' =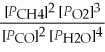
B)Kp' =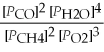
C)Kp' =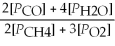
D)Kp' =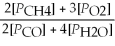
A)Kp' =
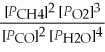
B)Kp' =
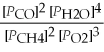
C)Kp' =
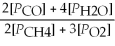
D)Kp' =
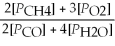
Kp' = 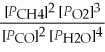
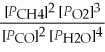
3
Which of the following statements is false regarding the equilibrium constant,Kc?
A)Kc for a reaction at a particular temperature always has the same value.
B)Kc for the reverse reaction is the negative of Kc for the forward reaction.
C)The numerical value of Kc depends on the form of the balanced equation.
D)When quoting Kc it is customary to omit units.
A)Kc for a reaction at a particular temperature always has the same value.
B)Kc for the reverse reaction is the negative of Kc for the forward reaction.
C)The numerical value of Kc depends on the form of the balanced equation.
D)When quoting Kc it is customary to omit units.
Kc for the reverse reaction is the negative of Kc for the forward reaction.
4
A mixture of carbon monoxide,hydrogen,and methanol is at equilibrium.The balanced chemical equation is: CO(g)+ 2 H2(g)⇌ CH3OH(g).At 250°C,the mixture contains 0.0960 M CO,0.191 M H2,and 0.150 M CH3OH.What is the value for Kc?
A)2.33 × 10-2
B)0.244
C)4.09
D)42.8
A)2.33 × 10-2
B)0.244
C)4.09
D)42.8

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
5
What is true about the relationship of Kp and Kc for the reaction: 2 CH4(g)+ 3 O2(g)⇌ 2 CO(g)+ 4 H2O(g)?
A)Kp < Kc
B)Kp = Kc
C)Kp > Kc
D)Kp and Kc are not related.
A)Kp < Kc
B)Kp = Kc
C)Kp > Kc
D)Kp and Kc are not related.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
6
Which statement about the equilibrium constant is true? The value of Kc
A)changes as product concentration changes.
B)changes as reactant concentration changes.
C)changes as temperature changes.
D)never changes.
A)changes as product concentration changes.
B)changes as reactant concentration changes.
C)changes as temperature changes.
D)never changes.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
7
If Kc is the equilibrium constant for a forward reaction,2 A⇌ B,what is Kc' for the reaction 4 A⇌ 2B?
A)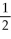
Kc
B)Kc
C)2 Kc
D)(Kc)2
A)
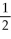
Kc
B)Kc
C)2 Kc
D)(Kc)2

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
8
The oxidation of sulfur dioxide by oxygen to sulfur trioxide has been implicated as an important step in the formation of acid rain: 2 SO2(g)+ O2(g)⇌ 2 SO3(g).If the equilibrium partial pressures of SO2,O2,and SO3 are 0.564 atm,0.102 atm,and 0.333 atm respectively at 1000 K,what is Kp at that temperature?
A)0.292
B)3.42
C)5.79
D)8.11
A)0.292
B)3.42
C)5.79
D)8.11

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
9
Which one of the following statements does not describe the equilibrium state?
A)Equilibrium is dynamic and there is no net conversion to reactants and products.
B)The concentration of the reactants is equal to the concentration of the products.
C)The concentration of the reactants and products reach a constant level.
D)The rate of the forward reaction is equal to the rate of the reverse reaction.
A)Equilibrium is dynamic and there is no net conversion to reactants and products.
B)The concentration of the reactants is equal to the concentration of the products.
C)The concentration of the reactants and products reach a constant level.
D)The rate of the forward reaction is equal to the rate of the reverse reaction.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
10
Kp = 1.5 × 103 at 400°C for the reaction 2 NH3(g)⇌ N2(g)+ 3 H2(g).What is the value of Kp for the reaction: 2 N2(g)+ 6 H2(g)⇌ 4 NH3(g)?
A)4.4 × 10-7
B)3.3 × 10-4
C)6.7 × 10-4
D)2.3 × 106
A)4.4 × 10-7
B)3.3 × 10-4
C)6.7 × 10-4
D)2.3 × 106

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
11
The equilibrium equation is also known as the law of
A)coefficients.
B)constant concentration.
C)dynamic equilibrium.
D)mass action.
A)coefficients.
B)constant concentration.
C)dynamic equilibrium.
D)mass action.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
12
Which statement about the equilibrium constant is true? The value of Kc
A)changes as product concentration changes.
B)changes as reactant concentration changes.
C)changes as temperature changes.
D)changes under the conditions described in A-C.
A)changes as product concentration changes.
B)changes as reactant concentration changes.
C)changes as temperature changes.
D)changes under the conditions described in A-C.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
13
Write the equilibrium equation for the forward reaction: 2 CH4(g)+ 3 O2(g)⇌ 2 CO(g)+ 4 H2O(g)
A)Kp =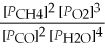
B)Kp=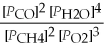
C)Kp =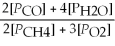
D)Kp =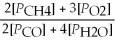
A)Kp =
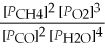
B)Kp=
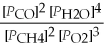
C)Kp =
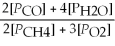
D)Kp =
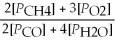

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
14
What is the equilibrium equation for the reaction: NH4NO3(s)⇌ N2O(g)+ 2 H2O(g)?
A)Kp = [N2O]
B)Kp = [N2O][H2O]
C)Kp = [N2O][H2O]2
D)Kp =![<strong>What is the equilibrium equation for the reaction: NH<sub>4</sub>NO<sub>3</sub>(s)⇌ N<sub>2</sub>O(g)+ 2 H<sub>2</sub>O(g)?</strong> A)K<sub>p</sub> = [N<sub>2</sub>O] B)K<sub>p</sub> = [N<sub>2</sub>O][H<sub>2</sub>O] C)K<sub>p</sub> = [N<sub>2</sub>O][H<sub>2</sub>O]<sup>2</sup> D)K<sub>p</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d164_226d_a2f7_e1d76e921017_TB4940_11.jpg)
A)Kp = [N2O]
B)Kp = [N2O][H2O]
C)Kp = [N2O][H2O]2
D)Kp =
![<strong>What is the equilibrium equation for the reaction: NH<sub>4</sub>NO<sub>3</sub>(s)⇌ N<sub>2</sub>O(g)+ 2 H<sub>2</sub>O(g)?</strong> A)K<sub>p</sub> = [N<sub>2</sub>O] B)K<sub>p</sub> = [N<sub>2</sub>O][H<sub>2</sub>O] C)K<sub>p</sub> = [N<sub>2</sub>O][H<sub>2</sub>O]<sup>2</sup> D)K<sub>p</sub> =](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d164_226d_a2f7_e1d76e921017_TB4940_11.jpg)

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
15
If Kc = 7.04 × 10-2 for the reaction: 2 HBr(g)⇌ H2(g)+ Br2(g),what is the value of Kc for the reaction: 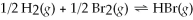 ?
?
A)3.52 × 10-2
B)0.265
C)3.77
D)28.4
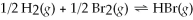 ?
?A)3.52 × 10-2
B)0.265
C)3.77
D)28.4

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
16
For the reaction: N2(g)+ 2 O2(g)⇌ 2 NO2(g),Kc = 8.3 × 10-10 at 25°C.What is the concentration of N2 gas at equilibrium when the concentration of NO2 is twice the concentration of O2 gas?
A)2.1 × 10-10 M
B)4.2 × 10-10 M
C)2.4 × 109 M
D)4.8 × 109 M
A)2.1 × 10-10 M
B)4.2 × 10-10 M
C)2.4 × 109 M
D)4.8 × 109 M

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
17
Given the reaction: 2 HI ⇌ H2 + I2.If Kc' for the reverse reaction is 1.85 × 10-2 at 425°C,what is Kc for the forward reaction at the same temperature?
A)-1.85 × 10-2
B)1.85 × 10-2
C)3.70 × 10-2
D)54.1
A)-1.85 × 10-2
B)1.85 × 10-2
C)3.70 × 10-2
D)54.1

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
18
Which one of the following statements about the equilibrium constant,Kp,is false?
A)Δn is equal to the sum of the coefficients of the gaseous products minus the sum of the coefficients of the gaseous reactants.
B)The relationship between Kp and Kc is: Kp = Kc (RT)Δn
C)The units for Kp are usually omitted.
D)Total pressures are used in the equilibrium equation in place of molar concentrations.
A)Δn is equal to the sum of the coefficients of the gaseous products minus the sum of the coefficients of the gaseous reactants.
B)The relationship between Kp and Kc is: Kp = Kc (RT)Δn
C)The units for Kp are usually omitted.
D)Total pressures are used in the equilibrium equation in place of molar concentrations.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
19
Nitric oxide reacts with oxygen to form nitrogen dioxide: 2 NO(g)+ O2(g)⇌ 2 NO2(g)
What is Kc' for the reverse reaction if the equilibrium concentration of NO is 0.300 M,O2 is 0.200 M,and NO2 is 0.530 M at 25°C?
A)0.0340
B)0.0641
C)0.624
D)15.6
What is Kc' for the reverse reaction if the equilibrium concentration of NO is 0.300 M,O2 is 0.200 M,and NO2 is 0.530 M at 25°C?
A)0.0340
B)0.0641
C)0.624
D)15.6

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
20
Kp is related to Kc by the equation Kp = Kc (RT)n.What is the value of n for the reaction below? NH4NO3(s)⇌ N2O(g)+ 2 H2O(g)
A)-2
B)-1
C)+1
D)+2
A)-2
B)-1
C)+1
D)+2

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
21
Phosphorus pentachloride decomposes to phosphorus trichloride and chlorine gas at elevated temperatures by the following reaction: PCl5(g)⇌ PCl3(g)+ Cl2(g).If Kc = 1.8 at 250°C.
What is the value of Kp at the same temperature?
A)4.2 × 10-2
B)8.8 × 10-2
C)65
D)77
What is the value of Kp at the same temperature?
A)4.2 × 10-2
B)8.8 × 10-2
C)65
D)77

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
22
The decomposition of ammonia is: 2 NH3(g)= N2(g)+ 3 H2(g).If Kp is 1.5 × 103 at 400°C,what is the partial pressure of ammonia at equilibrium when N2 is 0.10 atm and H2 is 0.15 atm?
A)2.2 × 10-7 atm
B)4.7 × 10-4 atm
C)2.1 × 103 atm
D)4.4 × 106 atm
A)2.2 × 10-7 atm
B)4.7 × 10-4 atm
C)2.1 × 103 atm
D)4.4 × 106 atm

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
23
What is the equilibrium equation for the dissociation of formic acid in water? HCOOH (aq)+ H2O (l)⇌ H3O+ (aq)+ HCOO- (aq)
A)Kc =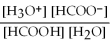
B)Kc =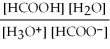
C)Kc =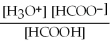
D)Kc =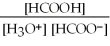
A)Kc =
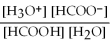
B)Kc =
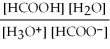
C)Kc =
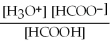
D)Kc =
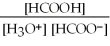

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
24
The equilibrium constant,Kp,equals 3.40 at 25°C for the isomerization reaction: cis-2-butene ⇌ trans-2-butene.
If a flask initially contains 1.00 atm of each gas,in what direction will the system shift to reach equilibrium?
A)It will shift left.
B)It will shift right.
C)The system is already at equilibrium.
D)The system is not at equilibrium and will remain in an unequilibrated state.
If a flask initially contains 1.00 atm of each gas,in what direction will the system shift to reach equilibrium?
A)It will shift left.
B)It will shift right.
C)The system is already at equilibrium.
D)The system is not at equilibrium and will remain in an unequilibrated state.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
25
Given the hypothetical reaction: 2 A(s)+ x B(g)⇌ 3 C(g),Kp = 0.0105 and Kc = 0.45 at 250°C.What is the value of the coefficient x?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
26
What is the value for Kc for the following reaction: PbCl2(s)⇌ Pb2+(aq)+ 2 Cl-(aq),
If PbCl2(s)= 1.50 grams,[Pb2+] = 1.6 × 10-2 M and [Cl-] = 3.2 × 10-2 M at equilibrium? (The molar mass of PbCl2(s)is 278 g/mol and its density is 5.85 g/cm3. )
A)7.6 × 10-7
B)1.6 × 10-5
C)6.2 × 104
D)1.3 × 106
If PbCl2(s)= 1.50 grams,[Pb2+] = 1.6 × 10-2 M and [Cl-] = 3.2 × 10-2 M at equilibrium? (The molar mass of PbCl2(s)is 278 g/mol and its density is 5.85 g/cm3. )
A)7.6 × 10-7
B)1.6 × 10-5
C)6.2 × 104
D)1.3 × 106

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
27
What is the equilibrium equation for the following reaction? C2H4 (g)+ 3 O2 (g)⇌ 2 CO2 (g)+ 2 H2O (l)
A)Kp =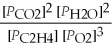
B)Kp =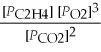
C)Kp =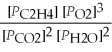
D)Kp =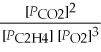
A)Kp =
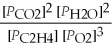
B)Kp =
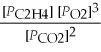
C)Kp =
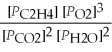
D)Kp =
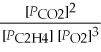

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
28
As a rule,which of the following phases are not included in the equilibrium constant expression? I.pure liquids II.pure solids III.aqueous solutions IV.gases
A)I,II
B)I,IV
C)III,IV
D)II,III
A)I,II
B)I,IV
C)III,IV
D)II,III

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
29
A 1.50 L vessel contains an equilibrium mixture of 0.100 mol of NO,0.150 mol of Br2,and 0.250 mol of NOBr at 25°C.What is the value of Kp for the reaction below? 2 NO(g)+ Br2(g)⇌ 2 NOBr(g)
A)2.56
B)62.5
C)1.28 × 102
D)1.53 × 103
A)2.56
B)62.5
C)1.28 × 102
D)1.53 × 103

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
30
At a certain temperature,bromine and nitric oxide react to form nitrosyl bromide: Br2(g)+ 2 NO(g)⇌ 2 NOBr(g).
When 0.010 mol Br2 is mixed with 0.025 mol NO and 0.015 mol NOBr in a 2.50 L flask,the concentration of NOBr decreases.Which statement below is true?
A)Kc < 36
B)Kc > 36
C)Kc < 90
D)Kc > 90
When 0.010 mol Br2 is mixed with 0.025 mol NO and 0.015 mol NOBr in a 2.50 L flask,the concentration of NOBr decreases.Which statement below is true?
A)Kc < 36
B)Kc > 36
C)Kc < 90
D)Kc > 90

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is the correct equilibrium constant expression for this reaction? (Hint: check the reaction coefficients) Fe(s)+ O2(g)⇌ Fe2O3(s)
A)K =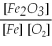
B)K =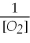
C)K =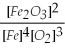
D)K =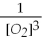
E)K = [O2}3
A)K =
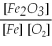
B)K =
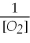
C)K =
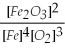
D)K =
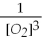
E)K = [O2}3

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
32
What is the equilibrium constant,Kc,for the reaction: 2 Hg(l)+ O2(g)⇌ 2 HgO(s)
If the amounts of reactants and products at equilibrium are: 1.00 g of HgO(s),2.00 mL of Hg(l),and 1.60 M O2(g)? (The molar mass and density of HgO(s)is 217 g/mol,and 1.10 g/cm3.The molar mass and density of Hg(l)is 201 g/mol,and 13.6 g/mL. )
A)6.85 × 10-4
B)4.66 × 10-2
C)0.625
D)1.46 × 103
If the amounts of reactants and products at equilibrium are: 1.00 g of HgO(s),2.00 mL of Hg(l),and 1.60 M O2(g)? (The molar mass and density of HgO(s)is 217 g/mol,and 1.10 g/cm3.The molar mass and density of Hg(l)is 201 g/mol,and 13.6 g/mL. )
A)6.85 × 10-4
B)4.66 × 10-2
C)0.625
D)1.46 × 103

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
33
The equilibrium constant is equal to 5.00 at 1300 K for the reaction: 2 SO2(g)+ O2(g)⇌ 2 SO3(g).
If initial concentrations are [SO2] = 1.20 M,[O2] = 0.45 M,and [SO3] = 1.80 M,the system is
A)at equilibrium.
B)not at equilibrium and will remain in an unequilibrated state.
C)not at equilibrium and will shift to the left to achieve an equilibrium state.
D)not at equilibrium and will shift to the right to achieve an equilibrium state.
If initial concentrations are [SO2] = 1.20 M,[O2] = 0.45 M,and [SO3] = 1.80 M,the system is
A)at equilibrium.
B)not at equilibrium and will remain in an unequilibrated state.
C)not at equilibrium and will shift to the left to achieve an equilibrium state.
D)not at equilibrium and will shift to the right to achieve an equilibrium state.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
34
At a certain temperature,bromine and nitric oxide react to form nitrosyl bromide: Br2(g)+ 2 NO(g)⇌ 2 NOBr(g).
When initial amounts of Br2,NO,and NOBr are mixed,the concentration of NOBr increases.Which statement below is true?
A)Kc < Q
B)Kc > Q
C)Kc = Q
D)More information is needed to make a statement about Kc.
When initial amounts of Br2,NO,and NOBr are mixed,the concentration of NOBr increases.Which statement below is true?
A)Kc < Q
B)Kc > Q
C)Kc = Q
D)More information is needed to make a statement about Kc.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
35
What is the equilibrium equation for the following reaction? FeS(s)+ 2 H3O+ (aq)⇌ Fe2+(aq)+ H2S (aq)+ 2 H2O (l)
A)Kc =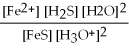
B)Kc =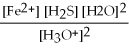
C)Kc =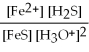
D)Kc =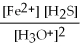
A)Kc =
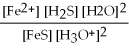
B)Kc =
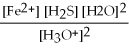
C)Kc =
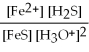
D)Kc =
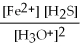

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
36
Cyclohexane (C6H12)undergoes a molecular rearrangement in the presence of AlCl3 to form methylcyclopentane (MCP)according to the equation: C6H12 ⇌ MCP
If Kc = 0.143 at 25°C for this reaction,predict the direction the reaction will shift if the initial concentrations of C6H12 and MCP are 0.200 M and 0.100 M,respectively.The system
A)will shift left.
B)will shift right.
C)is already at equilibrium.
D)is not at equilibrium and will remain in an unequilibrated state.
If Kc = 0.143 at 25°C for this reaction,predict the direction the reaction will shift if the initial concentrations of C6H12 and MCP are 0.200 M and 0.100 M,respectively.The system
A)will shift left.
B)will shift right.
C)is already at equilibrium.
D)is not at equilibrium and will remain in an unequilibrated state.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
37
Given the reaction at a certain temperature: 2 HI(g)⇌ H2(g)+ I2(g).At equilibrium,the partial pressure of HI is 1.8 × 10-3 atm,and the partial pressures for H2 and I2 are 0.10 atm each.Find Kp at that temperature.
A)3.2 × 10-4
B)5.6 × 101
C)3.1 × 103
D)3.1 × 104
A)3.2 × 10-4
B)5.6 × 101
C)3.1 × 103
D)3.1 × 104

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
38
If Kc = 2.0 × 1033 at 25°C,for the following reaction: H2(g)+ Cl2(g)⇌ 2 HCl(g),then find Kp at the same temperature.
A)8.2 × 1031
B)9.7× 1032
C)2.0 × 1033
D)4.9 × 1034
A)8.2 × 1031
B)9.7× 1032
C)2.0 × 1033
D)4.9 × 1034

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
39
What is the equilibrium equation for the following reaction? 2 H2O (l)⇌ H3O+ (aq)+ OH- (aq)
A)Kc =![<strong>What is the equilibrium equation for the following reaction? 2 H<sub>2</sub>O (l)⇌ H<sub>3</sub>O<sup>+</sup> (aq)+ OH<sup>-</sup> (aq)</strong> A)K<sub>c </sub>= B)K<sub>c </sub>= C)K<sub>c </sub>= D)K<sub>c </sub>= [H<sub>3</sub>O<sup>+</sup>] [OH<sup>-</sup>]](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d167_08b6_a2f7_1f1c4a40d437_TB4940_11.jpg)
B)Kc =![<strong>What is the equilibrium equation for the following reaction? 2 H<sub>2</sub>O (l)⇌ H<sub>3</sub>O<sup>+</sup> (aq)+ OH<sup>-</sup> (aq)</strong> A)K<sub>c </sub>= B)K<sub>c </sub>= C)K<sub>c </sub>= D)K<sub>c </sub>= [H<sub>3</sub>O<sup>+</sup>] [OH<sup>-</sup>]](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d167_08b7_a2f7_dd60ab52dde6_TB4940_11.jpg)
C)Kc =![<strong>What is the equilibrium equation for the following reaction? 2 H<sub>2</sub>O (l)⇌ H<sub>3</sub>O<sup>+</sup> (aq)+ OH<sup>-</sup> (aq)</strong> A)K<sub>c </sub>= B)K<sub>c </sub>= C)K<sub>c </sub>= D)K<sub>c </sub>= [H<sub>3</sub>O<sup>+</sup>] [OH<sup>-</sup>]](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d167_08b8_a2f7_13c3c6b84d25_TB4940_11.jpg)
D)Kc = [H3O+] [OH-]
A)Kc =
![<strong>What is the equilibrium equation for the following reaction? 2 H<sub>2</sub>O (l)⇌ H<sub>3</sub>O<sup>+</sup> (aq)+ OH<sup>-</sup> (aq)</strong> A)K<sub>c </sub>= B)K<sub>c </sub>= C)K<sub>c </sub>= D)K<sub>c </sub>= [H<sub>3</sub>O<sup>+</sup>] [OH<sup>-</sup>]](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d167_08b6_a2f7_1f1c4a40d437_TB4940_11.jpg)
B)Kc =
![<strong>What is the equilibrium equation for the following reaction? 2 H<sub>2</sub>O (l)⇌ H<sub>3</sub>O<sup>+</sup> (aq)+ OH<sup>-</sup> (aq)</strong> A)K<sub>c </sub>= B)K<sub>c </sub>= C)K<sub>c </sub>= D)K<sub>c </sub>= [H<sub>3</sub>O<sup>+</sup>] [OH<sup>-</sup>]](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d167_08b7_a2f7_dd60ab52dde6_TB4940_11.jpg)
C)Kc =
![<strong>What is the equilibrium equation for the following reaction? 2 H<sub>2</sub>O (l)⇌ H<sub>3</sub>O<sup>+</sup> (aq)+ OH<sup>-</sup> (aq)</strong> A)K<sub>c </sub>= B)K<sub>c </sub>= C)K<sub>c </sub>= D)K<sub>c </sub>= [H<sub>3</sub>O<sup>+</sup>] [OH<sup>-</sup>]](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d167_08b8_a2f7_13c3c6b84d25_TB4940_11.jpg)
D)Kc = [H3O+] [OH-]

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
40
The decomposition of ammonia is: 2 NH3(g)⇌ N2(g)+ 3 H2(g).If the pressure of ammonia is 1.0 × 10-3 atm,and the pressures of N2 and H2 are each 0.20 atm,what is the value for Kp' at 400°C for the reverse reaction?
A)-6.2 × 10-4
B)-1.6 × 103
C)6.2 × 10-4
D)1.6 × 103
A)-6.2 × 10-4
B)-1.6 × 103
C)6.2 × 10-4
D)1.6 × 103

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
41
When baking soda is heated it decomposes according to the following reaction: 2 NaHCO3(s)⇌ Na2CO3(s)+ H2O(g)+ CO2(g)
If sufficient baking soda is placed in a container and heated to 90°C,the total pressure of the gases is 0.5451 atm.What is the value of Kp at that temperature?
A)0.07428
B)0.2973
C)0.4228
D)1.091
If sufficient baking soda is placed in a container and heated to 90°C,the total pressure of the gases is 0.5451 atm.What is the value of Kp at that temperature?
A)0.07428
B)0.2973
C)0.4228
D)1.091

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
42
At a certain temperature the equilibrium constant,Kc,equals 0.11 for the reaction: 2 ICl(g)⇌ I2(g)+ Cl2(g).
What is the equilibrium concentration of ICl if 0.75 mol of I2 and 0.75 mol of Cl2 are initially mixed in a 2.0-L flask?
A)0.23 M
B)0.28 M
C)0.45 M
D)0.56
What is the equilibrium concentration of ICl if 0.75 mol of I2 and 0.75 mol of Cl2 are initially mixed in a 2.0-L flask?
A)0.23 M
B)0.28 M
C)0.45 M
D)0.56

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
43
For acid solutions of the same molarity acid strength is proportional to the equilibrium concentration of H3O+.For equimolar solutions of acids,which equilibrium expression below corresponds to the strongest acid?
A)Kc =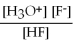
= 3.5 × 10-4
B)Kc =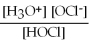
= 3.5 × 10-8
C)Kc =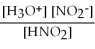
= 4.5 × 10-4
D)Kc =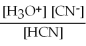
= 4.9 × 10-10
A)Kc =
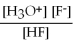
= 3.5 × 10-4
B)Kc =
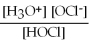
= 3.5 × 10-8
C)Kc =
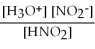
= 4.5 × 10-4
D)Kc =
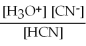
= 4.9 × 10-10

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
44
Cyclohexane (C6H12)undergoes a molecular rearrangement in the presence of AlCl3 to form methylcyclopentane (CH3C5H9)according to the equation: C6H12 ⇌ CH3C5H9
If Kc = 0.143 at 25°C for this reaction,find the equilibrium concentrations of C6H12 and CH3C5H9 if the initial concentrations are 0.200 M and 0.100 M,respectively.
A)[C6H12] = 0.0625 and [CH3C5H9] = 0.062 M
B)[C6H12] = 0.138 and [CH3C5H9] = 0.162 M
C)[C6H12] = 0.262 M and [CH3C5H9] = 0.038 M
D)[C6H12] = 0.282 and [CH3C5H9] = 0.018 M
If Kc = 0.143 at 25°C for this reaction,find the equilibrium concentrations of C6H12 and CH3C5H9 if the initial concentrations are 0.200 M and 0.100 M,respectively.
A)[C6H12] = 0.0625 and [CH3C5H9] = 0.062 M
B)[C6H12] = 0.138 and [CH3C5H9] = 0.162 M
C)[C6H12] = 0.262 M and [CH3C5H9] = 0.038 M
D)[C6H12] = 0.282 and [CH3C5H9] = 0.018 M

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
45
Oxalic acid can donate two protons to water in successive reactions: (1)H2C2O4(aq)+ H2O(l)⇌ H3O+(aq)+ HC2O4-(aq)
(2)HC2O4-(aq)+ H2O(l)⇌ H3O+(aq)+ C2O42-(aq)
If Kc1 = 5.9 × 10-2 and Kc2 = 6.4 × 10-5 at 25°C,what is the value of Kc for reaction (3)?
(3)H2C2O4(aq)+ 2 H2O(l)⇌ 2 H3O+(aq)+ C2O42-(aq)
A)3.8 × 10-6
B)1.1 × 10-3
C)5.9 × 10-2
D)9.2 × 102
(2)HC2O4-(aq)+ H2O(l)⇌ H3O+(aq)+ C2O42-(aq)
If Kc1 = 5.9 × 10-2 and Kc2 = 6.4 × 10-5 at 25°C,what is the value of Kc for reaction (3)?
(3)H2C2O4(aq)+ 2 H2O(l)⇌ 2 H3O+(aq)+ C2O42-(aq)
A)3.8 × 10-6
B)1.1 × 10-3
C)5.9 × 10-2
D)9.2 × 102

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
46
Ammonium carbamate can dissociate into gases at 25°C according to the reaction: NH2COONH4(s)⇌ 2 NH3(g)+ CO2(g)
If sufficient ammonium carbamate is sealed in a flask,the total pressure will be 0.117 atm at equilibrium.What is the value of Kp at 25°C?
A)2.37 × 10-4
B)2.00 × 10-4
C)1.60 × 10-3
D)3.42 × 10-1
If sufficient ammonium carbamate is sealed in a flask,the total pressure will be 0.117 atm at equilibrium.What is the value of Kp at 25°C?
A)2.37 × 10-4
B)2.00 × 10-4
C)1.60 × 10-3
D)3.42 × 10-1

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
47
Gaseous hydrogen bromide decomposes at elevated temperatures according to the following equation: 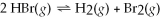 . At a certain temperature a 2.00 L flask is initially filled only with 0.600 mol of HBr.What is the value of Kc at that temperature if the flask contains 0.104 mol of H2 at equilibrium?
. At a certain temperature a 2.00 L flask is initially filled only with 0.600 mol of HBr.What is the value of Kc at that temperature if the flask contains 0.104 mol of H2 at equilibrium?
A)7.04 × 10-2
B)4.40 × 10-2
C)3.00 × 10-2
D)2.10 × 10-1
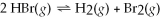 . At a certain temperature a 2.00 L flask is initially filled only with 0.600 mol of HBr.What is the value of Kc at that temperature if the flask contains 0.104 mol of H2 at equilibrium?
. At a certain temperature a 2.00 L flask is initially filled only with 0.600 mol of HBr.What is the value of Kc at that temperature if the flask contains 0.104 mol of H2 at equilibrium?A)7.04 × 10-2
B)4.40 × 10-2
C)3.00 × 10-2
D)2.10 × 10-1

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
48
Salt solubilities can be compared by the concentration of cation formed when the salt dissolves in the general reaction: MaXb(s)⇌ a Mb+(aq)+ b Xa-(aq).Given the following salts and their equilibrium constants for the reaction above at 25°C,which salt is the least soluble?
A)AgCl,Kc = 1.8 × 10-10
B)Ag2SO4,Kc = 1.2 × 10-5
C)CaCO3,Kc = 2.6 × 10-9
D)CaF2,Kc = 1.5 × 10-10
A)AgCl,Kc = 1.8 × 10-10
B)Ag2SO4,Kc = 1.2 × 10-5
C)CaCO3,Kc = 2.6 × 10-9
D)CaF2,Kc = 1.5 × 10-10

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
49
Phosphorus pentachloride decomposes to phosphorus trichloride at high temperatures according to the reaction: PCl5(g)⇌ PCl3(g)+ Cl2(g)
At 250°C,0.250 M PCl5 is added to a flask.If Kc = 1.80,what are the equilibrium concentrations of each gas?
A)[PCl5] = 0.0280 M,[PCl3] = 0.222 M,[Cl2] = 0.222 M
B)[PCl5] = 1.25 M,[PCl3] = 0.474 M,[Cl2] = 0.474 M
C)[PCl5] = 1.80 M,[PCl3] = 1.80 M,[Cl2] = 1.80 M
D)[PCl5] = 2.27 M,[PCl3] = 2.02 M,[Cl2] = 2.02 M
At 250°C,0.250 M PCl5 is added to a flask.If Kc = 1.80,what are the equilibrium concentrations of each gas?
A)[PCl5] = 0.0280 M,[PCl3] = 0.222 M,[Cl2] = 0.222 M
B)[PCl5] = 1.25 M,[PCl3] = 0.474 M,[Cl2] = 0.474 M
C)[PCl5] = 1.80 M,[PCl3] = 1.80 M,[Cl2] = 1.80 M
D)[PCl5] = 2.27 M,[PCl3] = 2.02 M,[Cl2] = 2.02 M

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
50
The equilibrium constant Kc for the reaction HF(aq)+ H2O(l)⇌ H3O+(aq)+F-(aq)is 3.5 × 10-4.What is the equilibrium concentration of H3O+ if the initial concentration of HF is 1.0 M?
A)1.0 M
B)3.5 × 10-2 M
C)1.9 × 10-2 M
D)1.9 × 10-4 M
A)1.0 M
B)3.5 × 10-2 M
C)1.9 × 10-2 M
D)1.9 × 10-4 M

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the reaction HCO3- (aq)+ H2O (l)⇌ CO3-2 (aq)+ H3O+ (aq)
The Keq for this reaction is 5.6 × 10-11.Describe what will happen to the reaction if the concentration of each reactant is
[HCO3-] = 5.6 × 10-11 [H3O+] = 1.2 × 10-11 [CO3-] = 5.6 × 10-11
A)Reaction will shift right,concentration of products will increase.
B)Reaction will shift left,concentration of reactants will increase.
C)Reaction will not change,it is at equilibrium.
D)Not enough information to determine the answer.
The Keq for this reaction is 5.6 × 10-11.Describe what will happen to the reaction if the concentration of each reactant is
[HCO3-] = 5.6 × 10-11 [H3O+] = 1.2 × 10-11 [CO3-] = 5.6 × 10-11
A)Reaction will shift right,concentration of products will increase.
B)Reaction will shift left,concentration of reactants will increase.
C)Reaction will not change,it is at equilibrium.
D)Not enough information to determine the answer.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
52
An equilibrium mixture of CO,O2 and CO2 at a certain temperature contains 0.0010 M CO2 and 0.0015 M O2.At this temperature,Kc,equals 1.4 × 102 for the reaction: 2 CO(g)+ O2(g)⇌ 2 CO2(g).
What is the equilibrium concentration of CO?
A)4.8 × 10-6 M
B)2.2 × 10-3 M
C)9.3 × 10-2 M
D)3.1 × 10-1 M
What is the equilibrium concentration of CO?
A)4.8 × 10-6 M
B)2.2 × 10-3 M
C)9.3 × 10-2 M
D)3.1 × 10-1 M

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
53
Kp is equal to 48.70 at 731 K for the reaction: H2(g)+ I2(g)⇌ 2 HI(g).Initially the mixture contains 0.08592 atm each of H2 and I2 and 1.0000 atm of HI.What is the pressure of HI at equilibrium?
A)0.7955 atm
B)0.9108 atm
C)0.9140 atm
D)0.9498 atm
A)0.7955 atm
B)0.9108 atm
C)0.9140 atm
D)0.9498 atm

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
54
At a certain temperature,Kc equals 1.4 × 102 for the reaction: 2 CO(g)+ O2(g)⇌ 2 CO2(g).
If a 2.50-L flask contains 0.400 mol of CO2 and 0.100 mol of O2 at equilibrium,how many moles of CO are also present in the flask?
A)0.422 mol
B)0.169 mol
C)0.107 mol
D)0.0114 mol
If a 2.50-L flask contains 0.400 mol of CO2 and 0.100 mol of O2 at equilibrium,how many moles of CO are also present in the flask?
A)0.422 mol
B)0.169 mol
C)0.107 mol
D)0.0114 mol

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
55
The solubility of 1:1 salts is measured by the equilibrium constant for the general reaction: MX(s)= Mn+(aq)+ Xn-(aq).Given the following salts and their equilibrium constants for the reaction above at 25°C,which salt is the least soluble?
A)MgCO3,Kc = 6.8 × 10-6
B)CaCO3,Kc = 5.0 × 10-9
C)SrCO3,Kc = 5.6 × 10-10
D)BaCO3,Kc = 2.6 × 10-9
A)MgCO3,Kc = 6.8 × 10-6
B)CaCO3,Kc = 5.0 × 10-9
C)SrCO3,Kc = 5.6 × 10-10
D)BaCO3,Kc = 2.6 × 10-9

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
56
The equilibrium constant,Kp,equals 3.40 for the isomerization reaction: cis-2-butene ⇌ trans-2-butene.
If a flask initially contains 0.250 atm of cis-2-butene and 0.125 atm of trans-2-butene,what is the equilibrium pressure of each gas?
A)P(cis-2-butene)= 0.037 atm,P(trans-2-butene)= 0.125 atm
B)P(cis-2-butene)= 0.048 atm,P(trans-2-butene)= 0.165 atm
C)P(cis-2-butene)= 0.074 atm,P(trans-2-butene)= 0.250 atm
D)P(cis-2-butene)= 0.085 atm,P(trans-2-butene)= 0.290 atm
If a flask initially contains 0.250 atm of cis-2-butene and 0.125 atm of trans-2-butene,what is the equilibrium pressure of each gas?
A)P(cis-2-butene)= 0.037 atm,P(trans-2-butene)= 0.125 atm
B)P(cis-2-butene)= 0.048 atm,P(trans-2-butene)= 0.165 atm
C)P(cis-2-butene)= 0.074 atm,P(trans-2-butene)= 0.250 atm
D)P(cis-2-butene)= 0.085 atm,P(trans-2-butene)= 0.290 atm

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
57
The esterification of acetic acid and ethanol is given by the reaction below: C2H5OH(aq)+ CH3COOH(aq)⇌ CH3COOC2H5(aq)+ H2O(l)
When 1.00 mol of ethanol was mixed with 2.00 mol of acid in a 1.00 L flask,0.86 mol of ester was formed at room temperature.What is the value of the equilibrium constant,Kc?
A)0.43
B)2.3
C)4.6
D)5.4
When 1.00 mol of ethanol was mixed with 2.00 mol of acid in a 1.00 L flask,0.86 mol of ester was formed at room temperature.What is the value of the equilibrium constant,Kc?
A)0.43
B)2.3
C)4.6
D)5.4

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
58
The following two isomers of C3H7NO exist in equilibrium with each other in solution: ![<strong>The following two isomers of C<sub>3</sub>H<sub>7</sub>NO exist in equilibrium with each other in solution: If K<sub>c</sub> = 0.57 at 25°C and the initial concentration of the reactant is 0.50 M and the product is 0.70 M,what are the concentrations at equilibrium?</strong> A)[reactant] = 0.43 M and [product] = 0.24 M B)[reactant] = 0.67 M and [product] = 0.38 M C)[reactant] = 0.76 M and [product] = 0.44 M D)[reactant] = 0.82 M and [product] = 0.47 M](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d168_1a29_a2f7_bf7192b07265_TB4940_00.jpg) If Kc = 0.57 at 25°C and the initial concentration of the reactant is 0.50 M and the product is 0.70 M,what are the concentrations at equilibrium?
If Kc = 0.57 at 25°C and the initial concentration of the reactant is 0.50 M and the product is 0.70 M,what are the concentrations at equilibrium?
A)[reactant] = 0.43 M and [product] = 0.24 M
B)[reactant] = 0.67 M and [product] = 0.38 M
C)[reactant] = 0.76 M and [product] = 0.44 M
D)[reactant] = 0.82 M and [product] = 0.47 M
![<strong>The following two isomers of C<sub>3</sub>H<sub>7</sub>NO exist in equilibrium with each other in solution: If K<sub>c</sub> = 0.57 at 25°C and the initial concentration of the reactant is 0.50 M and the product is 0.70 M,what are the concentrations at equilibrium?</strong> A)[reactant] = 0.43 M and [product] = 0.24 M B)[reactant] = 0.67 M and [product] = 0.38 M C)[reactant] = 0.76 M and [product] = 0.44 M D)[reactant] = 0.82 M and [product] = 0.47 M](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d168_1a29_a2f7_bf7192b07265_TB4940_00.jpg) If Kc = 0.57 at 25°C and the initial concentration of the reactant is 0.50 M and the product is 0.70 M,what are the concentrations at equilibrium?
If Kc = 0.57 at 25°C and the initial concentration of the reactant is 0.50 M and the product is 0.70 M,what are the concentrations at equilibrium?A)[reactant] = 0.43 M and [product] = 0.24 M
B)[reactant] = 0.67 M and [product] = 0.38 M
C)[reactant] = 0.76 M and [product] = 0.44 M
D)[reactant] = 0.82 M and [product] = 0.47 M

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
59
Acids donate protons to water according to the general equation: HA(aq)+ H2O(l)⇌ H3O+(aq)+ A-(aq)
Consider the following acids and their equilibrium constants for reaction with water at 25°C.If all the acids have the same initial concentration,which is the strongest acid (i.e.which donates the most protons to water)?
A)HBrO,Kc = 2.0 × 10-9
B)HNO2,Kc = 4.5 × 10-4
C)HF,Kc = 3.5 × 10-4
D)HIO3,Kc = 1.7 × 10-1
Consider the following acids and their equilibrium constants for reaction with water at 25°C.If all the acids have the same initial concentration,which is the strongest acid (i.e.which donates the most protons to water)?
A)HBrO,Kc = 2.0 × 10-9
B)HNO2,Kc = 4.5 × 10-4
C)HF,Kc = 3.5 × 10-4
D)HIO3,Kc = 1.7 × 10-1

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
60
For the isomerization reaction: butane ⇌ isobutane
Kp equals 25 at 500°C.If the initial pressures of butane and isobutane are 10.atm and 0.0 atm,respectively,what are the pressures of the two gases at equilibrium?
A)P(butane)= 0.38 atm and P(isobutane)= 9.6 atm
B)P(butane)= 0.40 atm and P(isobutane)= 10.atm
C)P(butane)= 9.6 atm and P(isobutane)= 0.38 atm
D)P(butane)= 10 atm and P(isobutane)= 0.40 atm
Kp equals 25 at 500°C.If the initial pressures of butane and isobutane are 10.atm and 0.0 atm,respectively,what are the pressures of the two gases at equilibrium?
A)P(butane)= 0.38 atm and P(isobutane)= 9.6 atm
B)P(butane)= 0.40 atm and P(isobutane)= 10.atm
C)P(butane)= 9.6 atm and P(isobutane)= 0.38 atm
D)P(butane)= 10 atm and P(isobutane)= 0.40 atm

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
61
The pink and blue species below form a violet colored mixture at equilibrium: [Co(H2O)6]2+ (aq)+ 4 Cl- (aq)⇌ [CoCl4]2- (aq)+ 6 H2O (l)
(pink)(blue)
If the concentration of [Co(H2O)6]2+ is increased,what happens to the solution?
A)The concentration of [CoCl4]2- increases.
B)The concentration of [CoCl4]2- decreases.
C)The solution becomes colorless.
D)No color change is observed.
(pink)(blue)
If the concentration of [Co(H2O)6]2+ is increased,what happens to the solution?
A)The concentration of [CoCl4]2- increases.
B)The concentration of [CoCl4]2- decreases.
C)The solution becomes colorless.
D)No color change is observed.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
62
Find the equilibrium constant for the reaction: A(g)+ B(g)⇌ 2C(g)at 25°C when k equals 1.4 × 10-12 M-1s-1 for the reaction A(g)+ B(g)→ 2C(g)at 25°C and k equals 2.7 × 10-13 M-1s-1 for the reaction: 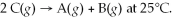
A)3.8 × 10-25
B)1.7 × 10-12
C)1.1 × 10-12
D)5.2
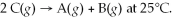
A)3.8 × 10-25
B)1.7 × 10-12
C)1.1 × 10-12
D)5.2

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
63
Consider the interconversion of A molecules (shaded spheres)and B molecules (unshaded spheres)according to the reaction A ⇌ B.Each of the following series of pictures represents a separate experiment in which time increases from left to right. 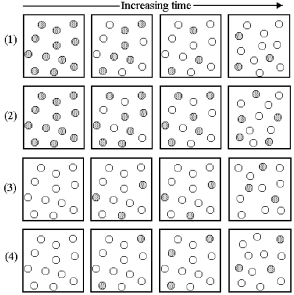
What is the value of the equilibrium constant Kc for the reaction A ⇌ B?
A)Kc = 0.33
B)Kc = 3.0
C)Kc = 12
D)Kc = 27

What is the value of the equilibrium constant Kc for the reaction A ⇌ B?
A)Kc = 0.33
B)Kc = 3.0
C)Kc = 12
D)Kc = 27

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
64
A catalyst increases the rate of a chemical reaction by providing a lower-energy mechanism for the reaction.When this occurs,which one of the following is not affected?
A)activation energy for the forward reaction
B)activation energy for the reverse reaction
C)equilibrium constant
D)rate of the reverse reaction
A)activation energy for the forward reaction
B)activation energy for the reverse reaction
C)equilibrium constant
D)rate of the reverse reaction

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
65
What effect will a change in temperature have on the value of Kp?
A)It will have no effect on the value of Kp.
B)The value of Kp always decreases with an increase in temperature.
C)The value of Kp always increases with an increase in temperature.
D)The value of Kp will decrease or increase with an increase in temperature,depending on whether the reaction is exothermic or endothermic.
A)It will have no effect on the value of Kp.
B)The value of Kp always decreases with an increase in temperature.
C)The value of Kp always increases with an increase in temperature.
D)The value of Kp will decrease or increase with an increase in temperature,depending on whether the reaction is exothermic or endothermic.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
66
The following pictures represent mixtures of cis-C2H2X2 molecules and trans-C2H2X2 molecules,which interconvert according to the equation cis-C2H2X2 ⇌ trans-C2H2X2.If mixture (1)is at equilibrium,which of the other mixtures are also at equilibrium? 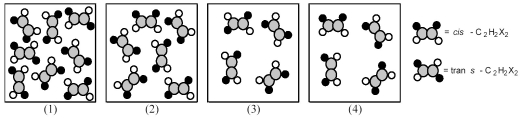
A)mixture (2)
B)mixture (3)
C)mixture (4)
D)None of the other mixtures are at equilibrium.

A)mixture (2)
B)mixture (3)
C)mixture (4)
D)None of the other mixtures are at equilibrium.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
67
The dissolution of calcium hydroxide is exothermic: Ca(OH)2(s)⇌ Ca2+(aq)+ 2 OH-(aq)
What happens when the solution of Ca(OH)2 is heated?
A)The amount of Ca(OH)2(s)decreases.
B)The amount of Ca(OH)2(s)increases.
C)The amount of Ca(OH)2(s)remains unchanged.
D)The Ca(OH)2(s)completely dissolves.
What happens when the solution of Ca(OH)2 is heated?
A)The amount of Ca(OH)2(s)decreases.
B)The amount of Ca(OH)2(s)increases.
C)The amount of Ca(OH)2(s)remains unchanged.
D)The Ca(OH)2(s)completely dissolves.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
68
Calcium carbonate is relatively insoluble and the dissolution reaction is endothermic: CaCO3(s)⇌ Ca2+(aq)+ CO32-(aq).
Which change in reaction condition below will shift the equilibrium to the right?
A)add an acid to react with CO32- ion
B)add an anion with which Ca2+ is even less soluble than calcium carbonate
C)increase the temperature
D)All of these will shift reaction to the right.
Which change in reaction condition below will shift the equilibrium to the right?
A)add an acid to react with CO32- ion
B)add an anion with which Ca2+ is even less soluble than calcium carbonate
C)increase the temperature
D)All of these will shift reaction to the right.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
69
A crude type of disappearing ink is based on the following endothermic equilibrium: [Co(H2O)6]Cl2 (aq)⇌ [CoCl2(H2O)4] (aq)+ 2 H2O (l)
(colorless)(blue)
If the reactant solution is used to write on a piece of paper and the paper is allowed to partially dry,what can be done to bring out the colored handwriting?
A)add water
B)decrease the volume
C)put the paper in a freezer
D)put the paper in an oven
(colorless)(blue)
If the reactant solution is used to write on a piece of paper and the paper is allowed to partially dry,what can be done to bring out the colored handwriting?
A)add water
B)decrease the volume
C)put the paper in a freezer
D)put the paper in an oven

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following will result in an increase in the amount of NH4Cl? NH4Cl (s)⇌ NH3 (g)+ HCl (g)
A)increasing the volume
B)decreasing the amount of HCl (g)
C)Iincreasing the amount of NH3 (g)
D)none of these
A)increasing the volume
B)decreasing the amount of HCl (g)
C)Iincreasing the amount of NH3 (g)
D)none of these

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
71
The hexaammine cobalt(III)ion is very unstable in acidic aqueous solution: [Co(NH3)6]3+(aq)+ 6 H3O+(aq)→ [Co(H2O)6]4+(aq)+ 6 NH4+(aq)
However,solutions of hexaammine cobalt(III)can be stored in acidic solution for months without noticeable decomposition.Which statement below about the equilibrium constant and the activation energy for the reaction is true?
A)Keq < 103 and Ea is very small.
B)Keq > 103 and Ea is very small.
C)Keq < 103 and Ea is very large.
D)Keq > 103 and Ea is very large.
However,solutions of hexaammine cobalt(III)can be stored in acidic solution for months without noticeable decomposition.Which statement below about the equilibrium constant and the activation energy for the reaction is true?
A)Keq < 103 and Ea is very small.
B)Keq > 103 and Ea is very small.
C)Keq < 103 and Ea is very large.
D)Keq > 103 and Ea is very large.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
72
Consider the interconversion of A molecules (shaded spheres)and B molecules (unshaded spheres)according to the reaction A ⇌ B.Each of the following series of pictures represents a separate experiment in which time increases from left to right. 
Which of these experiments has resulted in an equilibrium state?
A)all of the experiments except experiment (1)
B)all of the experiments except experiment (2)
C)all of the experiments except experiment (3)
D)all of the experiments except experiment (4)

Which of these experiments has resulted in an equilibrium state?
A)all of the experiments except experiment (1)
B)all of the experiments except experiment (2)
C)all of the experiments except experiment (3)
D)all of the experiments except experiment (4)

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
73
A reaction reaches dynamic equilibrium at a given temperature when
A)the amount of products exceeds the amount of reactants.
B)kfwd equals krev.
C)opposing reactions cease and the system is static.
D)the relative amounts of reactants and products are constant and ratefwd = raterev.
A)the amount of products exceeds the amount of reactants.
B)kfwd equals krev.
C)opposing reactions cease and the system is static.
D)the relative amounts of reactants and products are constant and ratefwd = raterev.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
74
The reaction A2 + B2 ⇌ 2 AB has an equilibrium constant Kc = 1.8.The following pictures represent reaction mixtures that contain A2 molecules (shaded)and B2 molecules (unshaded),and AB molecules.Which reaction mixture is at equilibrium? 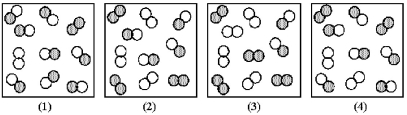
A)reaction mixture (1)
B)reaction mixture (2)
C)reaction mixture (3)
D)reaction mixture (4)

A)reaction mixture (1)
B)reaction mixture (2)
C)reaction mixture (3)
D)reaction mixture (4)

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
75
The following pictures represent the equilibrium state for four different reactions of the type 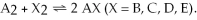 A atoms are unshaded.X atoms are shaded.
A atoms are unshaded.X atoms are shaded. 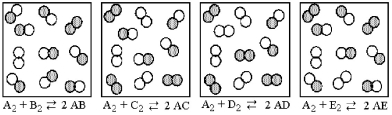
Which reaction has the largest equilibrium constant?
A)A2 + B2 ⇌ 2 AB
B)A2 + C2 ⇌ 2 AC
C)A2 + D2 ⇌ 2 AD
D)A2 + E2 ⇌ 2 AE
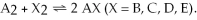 A atoms are unshaded.X atoms are shaded.
A atoms are unshaded.X atoms are shaded. 
Which reaction has the largest equilibrium constant?
A)A2 + B2 ⇌ 2 AB
B)A2 + C2 ⇌ 2 AC
C)A2 + D2 ⇌ 2 AD
D)A2 + E2 ⇌ 2 AE

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
76
Nickel metal can be prepared by the reduction of nickel oxide: NiO(s)+ CO(g)⇌ CO2(g)+ Ni(s)
At 936 K,Kp = 4.54 × 103 and at 1125 K,Kp = 1.58 × 103.Which statement is true?
A)The activation energy decreases with increasing temperature.
B)The activation energy increases with increasing temperature.
C)The reaction is endothermic.
D)The reaction is exothermic.
At 936 K,Kp = 4.54 × 103 and at 1125 K,Kp = 1.58 × 103.Which statement is true?
A)The activation energy decreases with increasing temperature.
B)The activation energy increases with increasing temperature.
C)The reaction is endothermic.
D)The reaction is exothermic.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
77
The reaction below virtually goes to completion because cyanide ion forms very stable complexes with Ni2+ ion: [Ni(H2O)6]2+(aq)+ 4 CN-(aq)→ [Ni(CN)4]2-(aq)+ 6 H2O(l)
At the same time,incorporation of 14C labelled cyanide ion (14CN-)is very rapid:
[Ni(CN)4]2-(aq)+ 4 14CN-(aq)= [Ni(14CN)4]2-(aq)+ 4 CN-(aq)
Which statement below is correct with regard to stability and rate of reaction?
A)Equilibrium is static.
B)Stable species can react rapidly.
C)Stable species do not react rapidly.
D)Unstable species react rapidly.
At the same time,incorporation of 14C labelled cyanide ion (14CN-)is very rapid:
[Ni(CN)4]2-(aq)+ 4 14CN-(aq)= [Ni(14CN)4]2-(aq)+ 4 CN-(aq)
Which statement below is correct with regard to stability and rate of reaction?
A)Equilibrium is static.
B)Stable species can react rapidly.
C)Stable species do not react rapidly.
D)Unstable species react rapidly.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
78
A catalyst increases the overall rate of reaction by lowering the activation energy,Ea,for
A)both the forward reaction and the reverse reaction.
B)neither the forward reaction nor the reverse reaction.
C)only the forward reaction.
D)only the reverse reaction.
A)both the forward reaction and the reverse reaction.
B)neither the forward reaction nor the reverse reaction.
C)only the forward reaction.
D)only the reverse reaction.

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
79
At 25°C,a certain first order reaction has a rate constant equal to 1.00 × 10-3 s-1 and an equilibrium constant,Kc,equal to 4.18.What is the rate constant for the reverse reaction?
A)2.39 × 10-4 s-1
B)4.18 × 10-3 s-1
C)2.39 × 102 s-1
D)4.18 × 103 s-1
A)2.39 × 10-4 s-1
B)4.18 × 10-3 s-1
C)2.39 × 102 s-1
D)4.18 × 103 s-1

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck
80
The following pictures represent the equilibrium state for four different reactions of the type 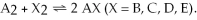 A atoms are unshaded.X atoms are shaded.
A atoms are unshaded.X atoms are shaded. 
Which reaction has the smallest equilibrium constant?
A)A2 + B2 ⇌ 2 AB
B)A2 + C2 ⇌ 2 AC
C)A2 + D2 ⇌ 2 AD
D)A2 + E2 ⇌ 2 AE
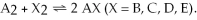 A atoms are unshaded.X atoms are shaded.
A atoms are unshaded.X atoms are shaded. 
Which reaction has the smallest equilibrium constant?
A)A2 + B2 ⇌ 2 AB
B)A2 + C2 ⇌ 2 AC
C)A2 + D2 ⇌ 2 AD
D)A2 + E2 ⇌ 2 AE

Unlock Deck
Unlock for access to all 171 flashcards in this deck.
Unlock Deck
k this deck



