Deck 12: Introduction to Organic Chemistry: Alkanes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/90
Play
Full screen (f)
Deck 12: Introduction to Organic Chemistry: Alkanes
1
The functional group illustrated by R-OH is an
A)alkyl.
B)alcohol.
C)ether.
D)ester.
E)aldehyde.
A)alkyl.
B)alcohol.
C)ether.
D)ester.
E)aldehyde.
alcohol.
2
Describe the unique characteristics of carbon that make it the basis of such a large number of organic compounds.
It has four valence electrons and is a very small atom,so it always forms four strong covalent bonds.It bonds with other carbon atoms to form long chains or rings.
3
Which atom is most likely to form a polar covalent bond with carbon?
A)C
B)H
C)Na
D)S
E)O
A)C
B)H
C)Na
D)S
E)O
O
4
Which family of organic molecules is a hydrocarbon?
A)alcohol
B)aldehyde
C)amide
D)amine
E)aromatic
A)alcohol
B)aldehyde
C)amide
D)amine
E)aromatic

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
5
All of the following statements are general properties of organic compounds except
A)the bonds are covalent.
B)they have relatively low boiling points.
C)they have limited or no water solubility.
D)they usually behave as electrolytes in solution.
E)they have relatively low melting points.
A)the bonds are covalent.
B)they have relatively low boiling points.
C)they have limited or no water solubility.
D)they usually behave as electrolytes in solution.
E)they have relatively low melting points.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
6
Which family of organic compounds does not contain any multiple bonds?
A)alkyl halides
B)alkenes
C)alkynes
D)aldehydes
E)ketones
A)alkyl halides
B)alkenes
C)alkynes
D)aldehydes
E)ketones

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
7
All of the families below include functional groups which contain oxygen except
A)carboxylic acids.
B)alkyl halides.
C)esters.
D)ethers.
E)ketones.
A)carboxylic acids.
B)alkyl halides.
C)esters.
D)ethers.
E)ketones.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
8
Which functional group does not contain oxygen?
A)alcohol
B)amine
C)carboxylic acid
D)ester
E)ketone
A)alcohol
B)amine
C)carboxylic acid
D)ester
E)ketone

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
9
The alcohol functional group has
A)a carbon-carbon triple bond.
B)a carbon-oxygen-hydrogen group.
C)a six-membered ring with three double bonds.
D)a double bond between carbon and oxygen.
E)one or more bonds between carbon and nitrogen.
A)a carbon-carbon triple bond.
B)a carbon-oxygen-hydrogen group.
C)a six-membered ring with three double bonds.
D)a double bond between carbon and oxygen.
E)one or more bonds between carbon and nitrogen.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
10
The functional group illustrated below is an 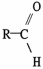
A)alkyl.
B)alcohol.
C)ether.
D)ester.
E)aldehyde.
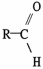
A)alkyl.
B)alcohol.
C)ether.
D)ester.
E)aldehyde.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
11
The aromatic functional group is often represented as
A)a carbon-carbon triple bond.
B)a carbon-oxygen-hydrogen group.
C)a six-membered ring with three double bonds.
D)at least one double bond between carbon and oxygen.
E)one or more bonds between carbon and nitrogen.
A)a carbon-carbon triple bond.
B)a carbon-oxygen-hydrogen group.
C)a six-membered ring with three double bonds.
D)at least one double bond between carbon and oxygen.
E)one or more bonds between carbon and nitrogen.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
12
Which atom is least likely to form a polar covalent bond with carbon?
A)Cl
B)F
C)H
D)N
E)O
A)Cl
B)F
C)H
D)N
E)O

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
13
The functional group illustrated by R-O-R' is an
A)alkyl.
B)alcohol.
C)ether.
D)ester.
E)aldehyde.
A)alkyl.
B)alcohol.
C)ether.
D)ester.
E)aldehyde.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
14
Which factor is most important in determining the chemistry of an organic molecule?
A)the number of carbon-carbon bonds
B)the functional groups
C)the number of carbon-hydrogen bonds
D)the number of branches in the carbon chain
E)the melting point
A)the number of carbon-carbon bonds
B)the functional groups
C)the number of carbon-hydrogen bonds
D)the number of branches in the carbon chain
E)the melting point

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
15
The functional group which illustrates an ester is
A)R-C≡C-R'.
B)R-C-N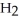
.
C)
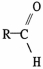
.
D)
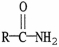
.
E)
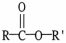
.
A)R-C≡C-R'.
B)R-C-N
.
C)
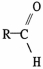
.
D)
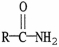
.
E)
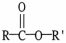
.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following statements is correct? Functional groups are
A)the parts of molecules that are used to differentiate types of organic molecules.
B)to organic chemistry what polyatomic ions are to inorganic chemistry.
C)are chemically bound to each other in order to make larger molecules.
D)There is more than one correct answer.
E)None are correct.
A)the parts of molecules that are used to differentiate types of organic molecules.
B)to organic chemistry what polyatomic ions are to inorganic chemistry.
C)are chemically bound to each other in order to make larger molecules.
D)There is more than one correct answer.
E)None are correct.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
17
The family of organic compounds with functional groups that do not consist only of hydrocarbons is
A)aldehydes.
B)alkanes.
C)alkenes.
D)alkynes.
E)aromatics.
A)aldehydes.
B)alkanes.
C)alkenes.
D)alkynes.
E)aromatics.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
18
The functional group illustrated below is an 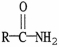
A)alcohol.
B)amide.
C)ether.
D)ester.
E)amine.
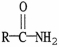
A)alcohol.
B)amide.
C)ether.
D)ester.
E)amine.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the statements about the behavior of the element carbon in organic molecules is incorrect?
A)Carbon can be involved in polar covalent bonds.
B)Carbon can form single,double,or triple bonds with other carbon atoms.
C)Carbon always forms four bonds.
D)When carbon forms four single bonds,the bond angles are 90°.
E)In addition to other carbon atoms,carbon is likely to form bonds with hydrogen,nitrogen,or oxygen.
A)Carbon can be involved in polar covalent bonds.
B)Carbon can form single,double,or triple bonds with other carbon atoms.
C)Carbon always forms four bonds.
D)When carbon forms four single bonds,the bond angles are 90°.
E)In addition to other carbon atoms,carbon is likely to form bonds with hydrogen,nitrogen,or oxygen.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
20
The alkyne functional group has
A)a carbon-carbon triple bond.
B)a carbon-oxygen-hydrogen group.
C)a six-membered ring with three double bonds.
D)a double bond between carbon and oxygen.
E)one or more bonds between carbon and nitrogen.
A)a carbon-carbon triple bond.
B)a carbon-oxygen-hydrogen group.
C)a six-membered ring with three double bonds.
D)a double bond between carbon and oxygen.
E)one or more bonds between carbon and nitrogen.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
21
Convert the following line structures to condensed formulas:
A)
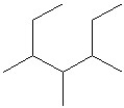
B)
A)

B)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is an isomer of the molecule shown below?
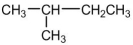
A)CH3CH2CH3
B)CH3CH(CH3)CH2CH3
C)CH3CH2CH2CH2CH3
D)CH3CH2CH2CH2CH2CH3

A)CH3CH2CH3
B)CH3CH(CH3)CH2CH3
C)CH3CH2CH2CH2CH3
D)CH3CH2CH2CH2CH2CH3

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
23
What functional groups are present in the following structure? 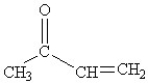
A)an alcohol and an alkene
B)a ketone and an alkyne
C)an aldehyde and a ketone
D)a ketone and an alkene
E)an ether and an alkane

A)an alcohol and an alkene
B)a ketone and an alkyne
C)an aldehyde and a ketone
D)a ketone and an alkene
E)an ether and an alkane

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
24
All of the following are representations of the same molecule except
A)C4H8
B)cyclobutane.
C)CH2CH2CH2CH2
.
D)
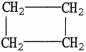
.
A)C4H8
B)cyclobutane.
C)CH2CH2CH2CH2
.
D)

.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
25
What functional groups are present in the following structure? 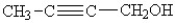
A)an alcohol and an alkyne
B)a ketone and an alkyne
C)an aldehyde and a ketone
D)an alcohol and an alkene
E)an ether and an alkyne
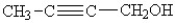
A)an alcohol and an alkyne
B)a ketone and an alkyne
C)an aldehyde and a ketone
D)an alcohol and an alkene
E)an ether and an alkyne

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is a correct line structure for the following molecule? 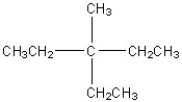
A)
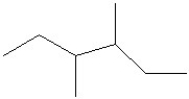
B)
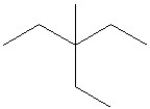
C)
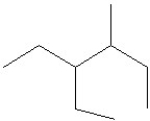
D)
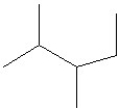

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
27
Explain the term "functional group." How do organic chemists use the functional group concept?

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
28
All of the choices listed are representations of the same molecule except
A) C5H12
B)CH3CH2CH2CH2CH3
C)
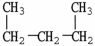
.
D)
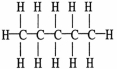
.
E)(CH3)2CHCH2CH3
.
A) C5H12
B)CH3CH2CH2CH2CH3
C)
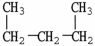
.
D)

.
E)(CH3)2CHCH2CH3
.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
29
The carbon skeleton of an alkane is shown below.How many hydrogen atoms are bonded to the carbon marked with a *? 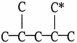
A)0
B)1
C)2
D)3
E)4
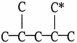
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
30
How many structural isomers are possible for the molecular formula,C4H10?
A)3
B)2
C)1
D)4
E)5
A)3
B)2
C)1
D)4
E)5

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
31
The carbon skeleton of an alkane is shown below.How many hydrogen atoms are bonded to the carbon marked with a *? 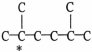
A)0
B)1
C)2
D)3
E)4
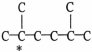
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
32
Which molecule is not an isomer of the molecule shown? 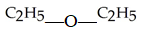
A)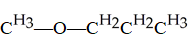
B) C4H9OH
C)
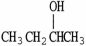
D)
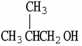
E)
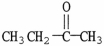
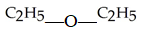
A)
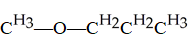
B) C4H9OH
C)

D)
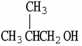
E)
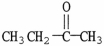

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following line structures are of the same compound? 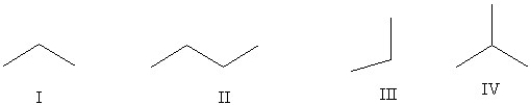
A)I and II
B)I and III
C)II and IV
D)All of them are the same.

A)I and II
B)I and III
C)II and IV
D)All of them are the same.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
34
Which list includes all the elements that would be found in an alkane with an amine group?
A)C,H
B)C,H,O
C)C,H,N
D)C,H,O,H
E)H,N
A)C,H
B)C,H,O
C)C,H,N
D)C,H,O,H
E)H,N

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
35
Which molecule is an isomer of the molecule shown? 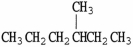
A)
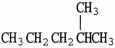
B)
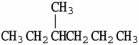
C)
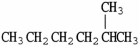
D)
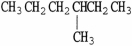
E)C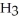
(C
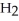
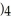
C
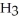
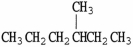
A)
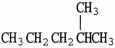
B)
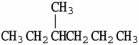
C)
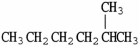
D)
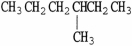
E)C
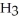
(C
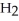
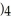
C
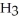

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
36
What are the two major functional groups in lactic acid? 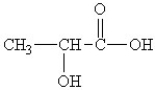
A)alkane and alcohol
B)carboxylic acid and alcohol
C)aldehyde and alcohol
D)ketone and diol
E)carboxylic acid and ether

A)alkane and alcohol
B)carboxylic acid and alcohol
C)aldehyde and alcohol
D)ketone and diol
E)carboxylic acid and ether

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
37
What is the relationship between the following molecules? 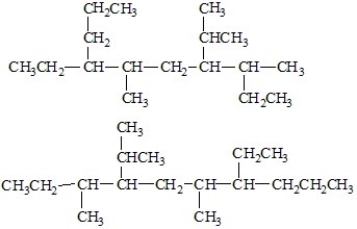
A)They are identical.
B)They are isomers of each other.
C)They are different molecules which are not isomers.
D)none of the above

A)They are identical.
B)They are isomers of each other.
C)They are different molecules which are not isomers.
D)none of the above

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
38
In straight-chain alkanes,the carbon atoms on each end of the molecule always form bonds with ________ atoms of hydrogen;the carbons within the molecule always form bonds with ________ hydrogen atoms.
A)4;4
B)4;2
C)3;3
D)3;2
E)2;2
A)4;4
B)4;2
C)3;3
D)3;2
E)2;2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
39
Two or more compounds with the same molecular formula but with the atoms connected differently are referred to as
A)normal alkanes.
B)branched alkanes.
C)functional groups.
D)constitutional isomers.
E)conformations.
A)normal alkanes.
B)branched alkanes.
C)functional groups.
D)constitutional isomers.
E)conformations.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following molecules contains both a carbonxylic acid group and an amine group?
A)
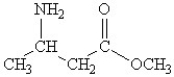
B)
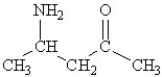
C)
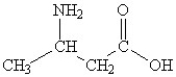
D)

E)
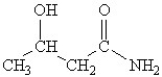
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
41
The various shapes taken on by an organic molecule are known as
A)conformations.
B)constitutional isomers.
C)preferential isomers.
D)configurations.
E)none of the above
A)conformations.
B)constitutional isomers.
C)preferential isomers.
D)configurations.
E)none of the above

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
42
Convert the following condensed formula to a line drawing: 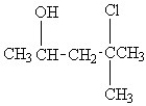
A)
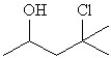
B)
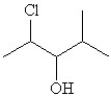
C)
D)
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
43
What is the IUPAC name of the compound shown?
(C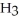
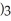 -C-C
-C-C 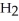 -C
-C 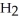 -C
-C 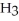
A)1,1,1-trimethylbutane
B)2,2-dimethylpentane
C)2-dimethylpentane
D)2-ethylhexane
E)heptane
(C
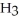
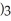 -C-C
-C-C 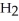 -C
-C 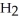 -C
-C 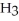
A)1,1,1-trimethylbutane
B)2,2-dimethylpentane
C)2-dimethylpentane
D)2-ethylhexane
E)heptane

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
44
The condensed structure of n-octane is
A)
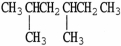
.
B)C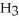 C
C 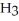 C
C 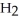 C
C 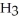 C
C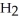 C
C 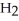
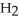 C
C 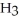
.
C)
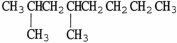
.
D)
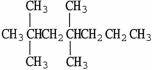
.
E)
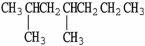
.
A)
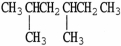
.
B)C
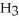 C
C 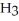 C
C 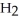 C
C 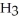 C
C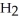 C
C 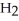
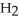 C
C 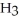
.
C)
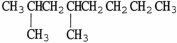
.
D)

.
E)
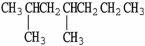
.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
45
Identify each pair of compounds as the same molecule,isomers or different molecules.
A)
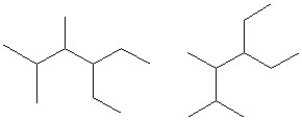
B)

C)
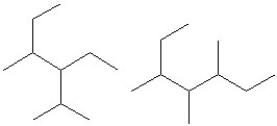
A)

B)

C)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
46
The name of the following structure will include the parent name 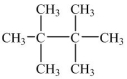
A)ethane.
B)butane.
C)hexane.
D)octane.
E)pentane.

A)ethane.
B)butane.
C)hexane.
D)octane.
E)pentane.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
47
The carbon skeleton of an alkane is shown below.How many hydrogen atoms are bonded to the carbon marked with a *? 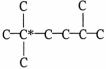
A)0
B)1
C)2
D)3
E)4

A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
48
How many carbon atoms are there in the longest continuous chain of the molecule shown?
(C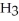

C-(C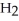
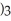
-C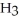
A)8
B)6
C)4
D)3
E)cannot be determined without additional information
(C
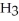

C-(C
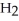
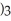
-C
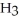
A)8
B)6
C)4
D)3
E)cannot be determined without additional information

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
49
The carbon atom marked with * is a ________ carbon atom. 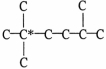
A)primary
B)secondary
C)tertiary
D)quaternary
E)none of these

A)primary
B)secondary
C)tertiary
D)quaternary
E)none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
50
The condensed structure of 2,2,4,4-tetramethylheptane is
A)
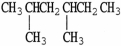
.
B)C C
C  C
C 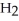 C
C 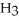 C
C 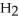 C
C 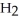 C
C 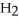 C
C 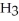
.
C)
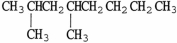
.
D)
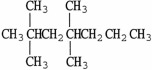
.
E)
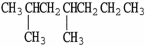
.
A)
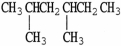
.
B)C
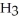 C
C 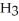 C
C 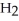 C
C 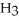 C
C 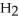 C
C 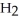 C
C 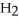 C
C 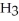
.
C)
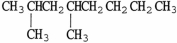
.
D)

.
E)
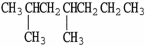
.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
51
What is the IUPAC name of the compound shown? 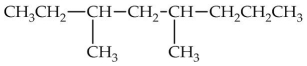
A)2-ethyl-4-methylheptane
B)4-methyl-2-ethylheptane
C)4,6-dimethyloctane
D)3,5-dimethyloctane
E)6-ethyl-4-methylheptane
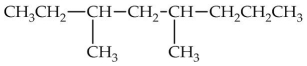
A)2-ethyl-4-methylheptane
B)4-methyl-2-ethylheptane
C)4,6-dimethyloctane
D)3,5-dimethyloctane
E)6-ethyl-4-methylheptane

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
52
What is the IUPAC name of the compound shown? 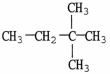
A)hexane
B)isohexane
C)ethylmethylpropane
D)dimethylbutane
E)2,2-dimethylbutane

A)hexane
B)isohexane
C)ethylmethylpropane
D)dimethylbutane
E)2,2-dimethylbutane

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
53
What is the IUPAC name of the compound shown? 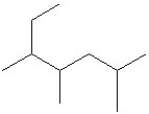
A)2-ethyl-3,5-dimethylhexane
B)3,5-dimethyl-2-ethylhexane
C)2,4,5-trimethylheptane
D)3,4,6-trimethylheptane
E)5-ethyl-2,4-dimethylhexane

A)2-ethyl-3,5-dimethylhexane
B)3,5-dimethyl-2-ethylhexane
C)2,4,5-trimethylheptane
D)3,4,6-trimethylheptane
E)5-ethyl-2,4-dimethylhexane

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
54
The carbon atom marked with * is a ________ carbon atom. 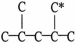
A)primary
B)secondary
C)tertiary
D)quaternary
E)none of these
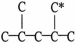
A)primary
B)secondary
C)tertiary
D)quaternary
E)none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following statements about alkyl groups is incorrect?
A)An alkyl group with four carbon atoms would include butyl in its name.
B)-C2H5 is an example.
C)In naming,they are used as prefixes and have a "yl" ending.
D)They are derived from alkenes.
E)none of the above
A)An alkyl group with four carbon atoms would include butyl in its name.
B)-C2H5 is an example.
C)In naming,they are used as prefixes and have a "yl" ending.
D)They are derived from alkenes.
E)none of the above

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
56
The name of the hydrocarbon with three carbon atoms and having only single bonds between carbon atoms is
A)decane.
B)butane.
C)propane.
D)ethane.
E)methane.
A)decane.
B)butane.
C)propane.
D)ethane.
E)methane.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
57
The carbon atom marked with * is a ________ carbon atom. 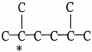
A)primary
B)secondary
C)tertiary
D)quaternary
E)none of these
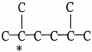
A)primary
B)secondary
C)tertiary
D)quaternary
E)none of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
58
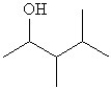
Select the line structure for the following condensed formula: 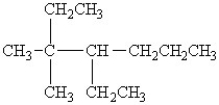
A)
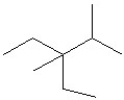
B)
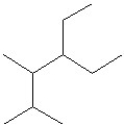
C)
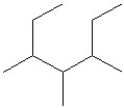
D)
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
59
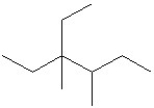
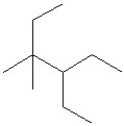
The condensed structure of 2,4-dimethylheptane is
A)
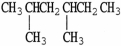
.
B)C C
C 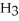 C
C 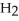
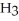 C
C 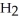 C
C 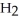 C
C 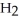 C
C 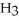
.
C)
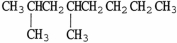
.
D)
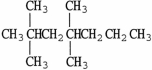
.
E)
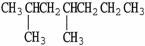
.
A)
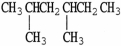
.
B)C
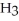 C
C 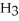 C
C 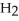
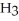 C
C 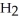 C
C 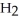 C
C 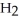 C
C 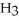
.
C)
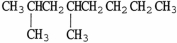
.
D)

.
E)
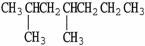
.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
60
What is the IUPAC name of the compound shown? 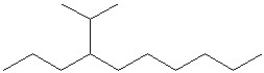
A)4-propyldecane
B)4-isopropyldecane
C)3-hexyl-2-methylhexane
D)4-isobutyldecane
E)4-sec-butyldecane

A)4-propyldecane
B)4-isopropyldecane
C)3-hexyl-2-methylhexane
D)4-isobutyldecane
E)4-sec-butyldecane

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
61
Write a line bond drawing for the following compounds:
A)1-chloro-2-isopropylcyclopentane
B) 1-ethyl-3-methylcyclohexane
A)1-chloro-2-isopropylcyclopentane
B) 1-ethyl-3-methylcyclohexane

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
62
Alkanes are ________ in water and ________ than water.
A)insoluble;less dense
B)soluble;less dense
C)insoluble,;more dense
D)soluble;more dense
A)insoluble;less dense
B)soluble;less dense
C)insoluble,;more dense
D)soluble;more dense

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
63
When hydrocarbons undergo complete combustion,the product(s)is(are)
A)CO2 and O2.
B)CO2.
C)CO2 and H2O.
D)H2O and O2.
E)H2O.
A)CO2 and O2.
B)CO2.
C)CO2 and H2O.
D)H2O and O2.
E)H2O.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
64
A correct name for the following compound is 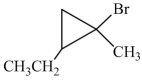
A)1-bromo-3-ethyl-1-methylcyclopropane.
B)1-bromo-2-ethyl-1-methylcyclopropane.
C)1-bromo-1-ethyl-2-methylcyclopropane.
D)2-bromo-1-ethyl-2-methylcyclopropane.
E)1-bromo-3-ethyl-3-methylcyclopropane.

A)1-bromo-3-ethyl-1-methylcyclopropane.
B)1-bromo-2-ethyl-1-methylcyclopropane.
C)1-bromo-1-ethyl-2-methylcyclopropane.
D)2-bromo-1-ethyl-2-methylcyclopropane.
E)1-bromo-3-ethyl-3-methylcyclopropane.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following properties is not characteristic of alkanes?
A)Their melting points increase with molecular weight.
B)They are generally less dense than water.
C)They are tasteless and colorless.
D)They are nontoxic.
E)They form strong hydrogen bonds.
A)Their melting points increase with molecular weight.
B)They are generally less dense than water.
C)They are tasteless and colorless.
D)They are nontoxic.
E)They form strong hydrogen bonds.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
66
Which substance is not reactive with respect to alkanes?
A)H2
B)Cl2
C)O2
D)Br2
E)none of the above
A)H2
B)Cl2
C)O2
D)Br2
E)none of the above

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
67
What is the minimum number of carbons that must be present in a molecule to have a secondary carbon?
A)3
B)1
C)2
D)4
E)5
A)3
B)1
C)2
D)4
E)5

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
68
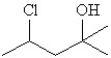
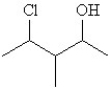
Which of the following is a correct line structure drawing for 3-ethyl-2,4-dimethylhexane?
A)
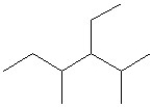
B)
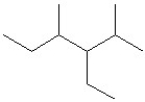
C)
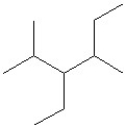
D)
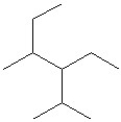
E)All answers are correct.
A)

B)

C)

D)

E)All answers are correct.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
69
When an alkane reacts with an element from group 7A,the reaction is referred to as
A)combustion.
B)decomposition.
C)displacement.
D)halogenation.
E)oxidation.
A)combustion.
B)decomposition.
C)displacement.
D)halogenation.
E)oxidation.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
70
How many hydrogens are present in a molecule composed of a six membered ring of carbon atoms and no double or triple bonds?
A)12
B)10
C)14
D)16
E)24
A)12
B)10
C)14
D)16
E)24

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
71
What is the IUPAC name of the molecule shown? 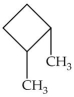
A)dimethylcyclobutane
B)cyclohexane
C)1,2-dimethylcyclobutane
D)2,3-dimethylcyclobutane
E)3,4-dimethylcyclobutane

A)dimethylcyclobutane
B)cyclohexane
C)1,2-dimethylcyclobutane
D)2,3-dimethylcyclobutane
E)3,4-dimethylcyclobutane

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
72
How many hydrogen atoms are present in the molecule shown? 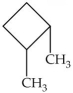
A)6
B)18
C)14
D)16
E)12

A)6
B)18
C)14
D)16
E)12

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
73
Which group is the best description of the properties of alkanes?
A)flammable,reactive,water soluble
B)non-flammable,non-polar,water soluble
C)flammable,non-reactive,insoluble in water
D)non-flammable,polar,reactive
E)none of the above
A)flammable,reactive,water soluble
B)non-flammable,non-polar,water soluble
C)flammable,non-reactive,insoluble in water
D)non-flammable,polar,reactive
E)none of the above

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
74
What is the IUPAC name of the compound shown? 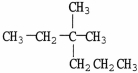
A)2-methyl-2-propylbutane
B)2-ethyl-2-methylpentane
C)2,2-ethyl-methylpentane
D)3,3-dimethylhexane
E)isooctane

A)2-methyl-2-propylbutane
B)2-ethyl-2-methylpentane
C)2,2-ethyl-methylpentane
D)3,3-dimethylhexane
E)isooctane

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
75
When hydrocarbons undergo incomplete combustion,the product(s)is(are)
A)CO2 and O2.
B)CO,H2O and soot.
C)CO2 and H2O.
D)H2O and O2.
E)H2O.
A)CO2 and O2.
B)CO,H2O and soot.
C)CO2 and H2O.
D)H2O and O2.
E)H2O.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
76
In the molecule 3,3-dimethylhexane,carbon number two is
A)primary.
B)secondary.
C)tertiary.
D)quaternary.
E)none of the above
A)primary.
B)secondary.
C)tertiary.
D)quaternary.
E)none of the above

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
77
The molecule shown is named as a substituted ________ because ________. 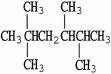
A)decane;it contains 10 atoms of carbon
B)hexane;it contains six atoms of carbon in its longest chain
C)tetramethane;it contains four methyl groups as branches
D)hexamethane;it contains six methyl groups altogether
E)butane;four carbons are substituted onto the chain

A)decane;it contains 10 atoms of carbon
B)hexane;it contains six atoms of carbon in its longest chain
C)tetramethane;it contains four methyl groups as branches
D)hexamethane;it contains six methyl groups altogether
E)butane;four carbons are substituted onto the chain

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
78
Which is not a property of most alkanes?
A)soluble in water
B)light odor
C)colorless
D)burn readily in an open flame
E)They are all properties of alkanes.
A)soluble in water
B)light odor
C)colorless
D)burn readily in an open flame
E)They are all properties of alkanes.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
79
What is the IUPAC name of the molecule shown? 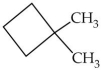
A)dimethylcyclobutane
B)cyclohexane
C)1,2-dimethylcyclobutane
D)1,1-dimethylcyclobutane
E)2,2-dimethylcyclobutane

A)dimethylcyclobutane
B)cyclohexane
C)1,2-dimethylcyclobutane
D)1,1-dimethylcyclobutane
E)2,2-dimethylcyclobutane

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
80
Monochlorination of pentane,C5H12,leads to formation of how many different products?
A)3
B)2
C)1
D)4
E)5
A)3
B)2
C)1
D)4
E)5

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck



