Deck 15: Chemical Kinetics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/88
Play
Full screen (f)
Deck 15: Chemical Kinetics
1
If the rate of reaction increases by a factor of 2.3 when the concentration of reactant increases by a factor of 1.5,the order of the reaction with respect to this reactant is
A)2.
B)1.
C)1.5.
D)4.
E)3.
A)2.
B)1.
C)1.5.
D)4.
E)3.
2.
2
The reaction 2NO(g)+ 2H2(g) N2(g)+ 2H2O(g)
Is first order in H2 and second order in NO.Starting with equal concentrations of H2 and NO,the rate after 50% of the H2 has reacted is what percent of the initial rate?
A)18.8%
B)37.5%
C)25.0%
D)50.0%
E)12.5%
Is first order in H2 and second order in NO.Starting with equal concentrations of H2 and NO,the rate after 50% of the H2 has reacted is what percent of the initial rate?
A)18.8%
B)37.5%
C)25.0%
D)50.0%
E)12.5%
12.5%
3
Given: 2O3(g) 3O2(g)
Rate = k[O3]2[O2] - 1
The overall order of the reaction and the order with respect to [O3] are
A)(-1 and 3.)
B)1 and 2.
C)0 and 1.
D)2 and 2.
E)3 and 2.
Rate = k[O3]2[O2] - 1
The overall order of the reaction and the order with respect to [O3] are
A)(-1 and 3.)
B)1 and 2.
C)0 and 1.
D)2 and 2.
E)3 and 2.
1 and 2.
4
The reaction 2NO(g)+ 2H2(g) N2(g)+ 2H2O(g)
Is first order in H2 and second order in NO.Starting with equal concentrations of H2 and NO,the rate after 25% of the H2 has reacted is what percent of the initial rate?
A)75.0%
B)42.2%
C)6.25%
D)56.3%
E)1.56%
Is first order in H2 and second order in NO.Starting with equal concentrations of H2 and NO,the rate after 25% of the H2 has reacted is what percent of the initial rate?
A)75.0%
B)42.2%
C)6.25%
D)56.3%
E)1.56%

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
5
If the rate of reaction increases by a factor of 9.6 when the concentration of reactant increases by a factor of 3.1,the order of the reaction with respect to this reactant is
A)1.5
B)3
C)4
D)2
E)1
A)1.5
B)3
C)4
D)2
E)1

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
6
The concentration-time dependence for a first-order reaction is: 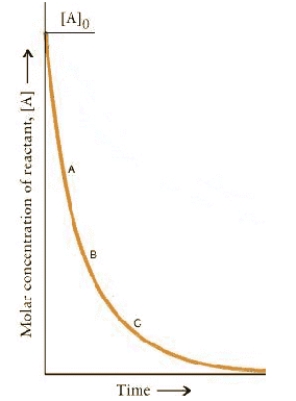 At which point on the curve is the reaction fastest?
At which point on the curve is the reaction fastest?
A)A
B)B
C)C
D)A + t½
E)The rates are the same at all points.
 At which point on the curve is the reaction fastest?
At which point on the curve is the reaction fastest?A)A
B)B
C)C
D)A + t½
E)The rates are the same at all points.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
7
If the average rate of formation of H2(g)is 3.90 (mol H2).L - 1.s - 1 for the reaction 2PH3(g) 2P(g)+ 3H2(g),the unique average reaction rate is
A)3.90 mol.L - 1.s - 1.
B)1.30 mol.L - 1.s - 1.
C)2.60 mol.L - 1.s - 1.
D)7.80 mol.L - 1.s - 1.
E)11.7 mol.L - 1.s - 1.
A)3.90 mol.L - 1.s - 1.
B)1.30 mol.L - 1.s - 1.
C)2.60 mol.L - 1.s - 1.
D)7.80 mol.L - 1.s - 1.
E)11.7 mol.L - 1.s - 1.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
8
If the average rate of decomposition of PH3(g)is 3.2 (mol PH3).L - 1.min - 1 for the reaction 2PH3(g) 2P(g)+ 3H2(g),the unique average reaction rate is
A)3.2 mol.L - 1.min - 1.
B)2.1 mol.L - 1.min - 1.
C)1.6 mol.L - 1.min sup>- 1.
D)4.8 mol.L - 1.min - 1.
E)6.4 mol.L - 1.min - 1.
A)3.2 mol.L - 1.min - 1.
B)2.1 mol.L - 1.min - 1.
C)1.6 mol.L - 1.min sup>- 1.
D)4.8 mol.L - 1.min - 1.
E)6.4 mol.L - 1.min - 1.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
9
Given: 4Fe2+(aq)+ O2(aq)+ 2H2O(l) 4Fe3+(aq)+ 4OH - (aq)
Rate = k[Fe2+][OH - ]2[O2]
The overall order of the reaction and the order with respect to O2 are
A)4 and 1.
B)5 and 1.
C)3 and 1.
D)4 and 2.
E)7 and 1.
Rate = k[Fe2+][OH - ]2[O2]
The overall order of the reaction and the order with respect to O2 are
A)4 and 1.
B)5 and 1.
C)3 and 1.
D)4 and 2.
E)7 and 1.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
10
If the rate of a reaction increases by a factor of 64 when the concentration of reactant increases by a factor of 4,the order of the reaction with respect to this reactant is
A)16.
B)2.
C)4.
D)1.
E)3.
A)16.
B)2.
C)4.
D)1.
E)3.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
11
The concentration-time dependence for two first order reactions is: 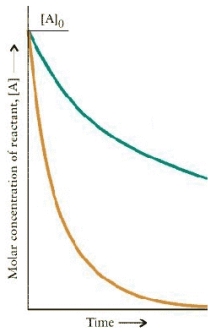 Which reaction has the greater t½?
Which reaction has the greater t½?
 Which reaction has the greater t½?
Which reaction has the greater t½?
Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
12
For the reaction S2O82 - (aq)+ 3I - (aq) 2SO42 - aq)+ I3-(aq),rate = k[S2O82 - ][I - ].When the reaction is followed under pseudo-first-order conditions with [S2O82 - ] = 200 m M and [ - ] = 1.5 m M,the rate constant was 1.82 s - 1.The second order rate constant,k,for the reaction is
A)1.21 * 103 M - 1.s - 1.
B)6.07* 103 M - 1.s - 1.
C)9.10 M - 1.s - 1.
D)1.37 *10-2 M - 1.s - 1.
E)1.82 M - 1.s - 1.
A)1.21 * 103 M - 1.s - 1.
B)6.07* 103 M - 1.s - 1.
C)9.10 M - 1.s - 1.
D)1.37 *10-2 M - 1.s - 1.
E)1.82 M - 1.s - 1.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
13
It is important to distinguish between the reaction rate and the rate constant.The units of reaction rate are M∙s - 1.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
14
The rate of formation of oxygen in the reaction 2N2O5(g) 4NO2(g)+ O2(g)
Is 2.28 (mol O2).L - 1F.s - 1.What is the rate of formation of NO2?
A)0.57 (mol NO2).L - 1.s - 1
B)9.12 (mol NO2).L - 1.s - 1
C)2.28 (mol NO2).L - 1.s - 1
D)1.14 (mol NO2).L - 1.s - 1
E)4.56 (mol NO2).L - 1.s - 1
Is 2.28 (mol O2).L - 1F.s - 1.What is the rate of formation of NO2?
A)0.57 (mol NO2).L - 1.s - 1
B)9.12 (mol NO2).L - 1.s - 1
C)2.28 (mol NO2).L - 1.s - 1
D)1.14 (mol NO2).L - 1.s - 1
E)4.56 (mol NO2).L - 1.s - 1

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
15
The concentration-time curves for two sets of reactions,A/B and C/D,are: 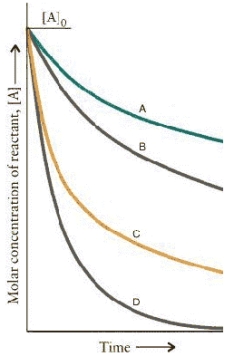 Which of the reactions are first order?
Which of the reactions are first order?
A)B and D
B)A and B
C)C and D
D)A and C
 Which of the reactions are first order?
Which of the reactions are first order?A)B and D
B)A and B
C)C and D
D)A and C

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
16
Given: 2A(g)+ B(g) C(g)+ D(g)
When [A] = [B] = 0.10 M,the rate is 2.0 M.s - 1; for [A] = [B] = 0.20,the rate is 8.0 M.s - 1; and for [A] = 0.10 M,[B] = 0.20 M,the rate is 2.0 M.s - 1.The rate law is
A)rate = k[A].
B)rate = k[B]2.
C)rate = k[A][B]0.
D)rate = k[A][B].
E)rate = k[A]2.
When [A] = [B] = 0.10 M,the rate is 2.0 M.s - 1; for [A] = [B] = 0.20,the rate is 8.0 M.s - 1; and for [A] = 0.10 M,[B] = 0.20 M,the rate is 2.0 M.s - 1.The rate law is
A)rate = k[A].
B)rate = k[B]2.
C)rate = k[A][B]0.
D)rate = k[A][B].
E)rate = k[A]2.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
17
A first-order reaction has a rate constant of 0.00300 s - 1.The time required for 60% reaction is
A)153 s.
B)73.9 s.
C)170 s.
D)133 s.
E)305 s.
A)153 s.
B)73.9 s.
C)170 s.
D)133 s.
E)305 s.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
18
The concentration-time curves for two sets of reactions,A/B and C/D,are: 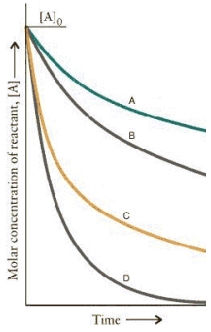 Which set of reactions has the largest rate constant?
Which set of reactions has the largest rate constant?
 Which set of reactions has the largest rate constant?
Which set of reactions has the largest rate constant?
Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
19
For a given first-order reaction,after 2.00 min,20% of the reactants remain.Calculate the rate constant for the reaction.
A)0.0134 s - 1
B)0.000808 s - 1
C)74.6 s - 1
D)0.00582 s - 1
E)0.00186 s - 1
A)0.0134 s - 1
B)0.000808 s - 1
C)74.6 s - 1
D)0.00582 s - 1
E)0.00186 s - 1

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
20
The concentration-time dependence is shown below for two first-order reactions is: 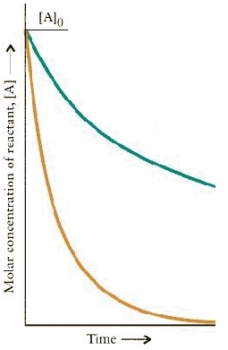 Which reaction has the larger rate constant?
Which reaction has the larger rate constant?
 Which reaction has the larger rate constant?
Which reaction has the larger rate constant?
Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
21
Given: A P rate = k[A] If 20% of A reacts in 5.12 min,calculate the time required for 90% of A to react.
A)52.8 min
B)1.05 min
C)2.42 min
D)3170 min
E)22.9 min
A)52.8 min
B)1.05 min
C)2.42 min
D)3170 min
E)22.9 min

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
22
For a second-order reaction,a straight line is obtained from a plot of
A)1/[A] vs t.
B)ln(1/t)vs [A].
C)[A] vs t.
D)ln[A] vs t.
E)ln(t)vs [A].
A)1/[A] vs t.
B)ln(1/t)vs [A].
C)[A] vs t.
D)ln[A] vs t.
E)ln(t)vs [A].

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
23
A nonsteroidal anti-inflammatory drug is metabolized with a first-order rate constant of 3.25 day - 1.What fraction of the drug remains in the body after 13.0 hr?
A)0.172
B)0.0174
C)0.828
D)0.873
E)0.127
A)0.172
B)0.0174
C)0.828
D)0.873
E)0.127

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
24
For the reaction cyclobutane(g) 2ethylene(g)at 800 K,a plot of ln[cyclobutane] vs t gives a straight line with a slope of-1.6 s - 1.Calculate the time needed for the concentration of cyclobutane to fall to 1/16 of its initial value.
A)2.3 s
B)1.7 s
C)1.3 s
D)0.63 s
E)1.6 s
A)2.3 s
B)1.7 s
C)1.3 s
D)0.63 s
E)1.6 s

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
25
A first-order reaction has a half-life of 1.10 s.If the initial concentration of reactant is 0.384 M,how long will it take for the reactant concentration to reach 0.00100 M?
A)9.45 s
B)0.106 s
C)4.10 s
D)1.52 s
E)0.244 s
A)9.45 s
B)0.106 s
C)4.10 s
D)1.52 s
E)0.244 s

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
26
Technetium-99m,used to image the heart and brain,has a half-life of 6.00 h.What fraction of technetium-99m remains in the body after 1 day?
A)0.0625
B)0.250
C)0.0313
D)0.125
A)0.0625
B)0.250
C)0.0313
D)0.125

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
27
For the reaction A products,the following data were collected. 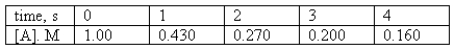 Determine the order of the reaction and calculate the rate constant.
Determine the order of the reaction and calculate the rate constant.
 Determine the order of the reaction and calculate the rate constant.
Determine the order of the reaction and calculate the rate constant. 
Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
28
For the reaction cyclopropane propene
A plot of ln[cyclopropane] vs time in seconds gives a straight line with slope -4.1 *10 - 3 s - 1 at 550 C.What is the rate constant for this reaction?
A)3.9 *10 - 2 s - 1
B)8.2 *10 - 3 s - 1
C)4.1 *10 - 3 s - 1
D)1.8 *10 - 3 s - 1
E)2.1 * 10 - 3 s - 1
A plot of ln[cyclopropane] vs time in seconds gives a straight line with slope -4.1 *10 - 3 s - 1 at 550 C.What is the rate constant for this reaction?
A)3.9 *10 - 2 s - 1
B)8.2 *10 - 3 s - 1
C)4.1 *10 - 3 s - 1
D)1.8 *10 - 3 s - 1
E)2.1 * 10 - 3 s - 1

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
29
A given first-order reaction has a rate constant of 0.00300 s - 1.The time required for 85% reaction is
A)632 s.
B)23.5 s.
C)275 s.
D)316 s.
E)54.2 s.
A)632 s.
B)23.5 s.
C)275 s.
D)316 s.
E)54.2 s.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
30
The rates of first-order and second-order reactions do not change with elapsed time.True or false?

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
31
For a given first-order reaction,after 230 s,33% of the reactants remain.Calculate the rate constant for the reaction.
A)207 s - 1
B)0.00174 s - 1
C)0.00209 s - 1
D)0.000756 s - 1
E)0.00482 s - 1
A)207 s - 1
B)0.00174 s - 1
C)0.00209 s - 1
D)0.000756 s - 1
E)0.00482 s - 1

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
32
What is the rate constant for a first-order reaction with a half-life of 9.0 ms?
A)13 s - 1
B)77 s - 1
C)9.0 s - 1
D)6.2 s - 1
E)0.11 s - 1
A)13 s - 1
B)77 s - 1
C)9.0 s - 1
D)6.2 s - 1
E)0.11 s - 1

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
33
Consider the reaction 2N2O(g) 2N2(g)+ O2(g)
Rate = k[N2O]
For an initial concentration of N2O of 0.50 M,what is the concentration of N2O remaining after 2.0 min if k = 3.4*10 - 3 s - 1?
A)0.50 M
B)0.55 M
C)0.66 M
D)0.33 M
E)0.17 M
Rate = k[N2O]
For an initial concentration of N2O of 0.50 M,what is the concentration of N2O remaining after 2.0 min if k = 3.4*10 - 3 s - 1?
A)0.50 M
B)0.55 M
C)0.66 M
D)0.33 M
E)0.17 M

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the reaction 2N2O5(g) 4NO2(g)+ O2(g)
Rate = k[N2O5]
Calculate the time for the concentration of N2O5 to fall to one-fourth its initial value if the half-life is 133 s.
A)133 s
B)33.3 s
C)266 s
D)66.6 s
E)533 s
Rate = k[N2O5]
Calculate the time for the concentration of N2O5 to fall to one-fourth its initial value if the half-life is 133 s.
A)133 s
B)33.3 s
C)266 s
D)66.6 s
E)533 s

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
35
For the reaction cyclopropane(g) propene(g)at 500 C,a plot of ln[cyclopropane] vs t gives a straight line with a slope of -0.00067 s - 1.What is the order of this reaction and what is the rate constant?

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
36
Consider the reaction 2N2O(g) 2N2(g)+ O2(g)
Rate = k[N2O]
Calculate the time required for the concentration of N2O(g)to decrease from 0.75 M to 0.33 M.The rate constant for the reaction is k = 6.8 *10 - 3 s - 1.
A)2.7 min
B)1.7 min
C)0.87 min
D)0.92 min
E)2.0 min
Rate = k[N2O]
Calculate the time required for the concentration of N2O(g)to decrease from 0.75 M to 0.33 M.The rate constant for the reaction is k = 6.8 *10 - 3 s - 1.
A)2.7 min
B)1.7 min
C)0.87 min
D)0.92 min
E)2.0 min

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
37
A given compound decomposes with a half-life of 8.0 s and the half-life is independent of the concentration.How long does it take for the concentration to decrease to one-ninth of its initial value?
A)32 s
B)25 s
C)72 s
D)64 s
E)3.6 s
A)32 s
B)25 s
C)72 s
D)64 s
E)3.6 s

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
38
The reaction 2ClO2(g)+ F2(g 2FClO2(g)
Is first order in both ClO2 and F2.When the initial concentrations of ClO2 and F2 are equal,the rate after 25% of the F2 has reacted is what percent of the initial rate?
A)75.0%
B)18.8%
C)37.5%
D)28.1%
E)12.5%
Is first order in both ClO2 and F2.When the initial concentrations of ClO2 and F2 are equal,the rate after 25% of the F2 has reacted is what percent of the initial rate?
A)75.0%
B)18.8%
C)37.5%
D)28.1%
E)12.5%

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
39
Consider the reaction 2N2O5(g) 4NO2(g)+ O2(g)
Rate = k[N2O5]
If the initial concentration of N2O5 is 0.80 M,the concentration after 5 half-lives is
A)0.11 M.
B)0.025 M.
C)0.032 M.
D)0.16 M.
E)0.050 M.
Rate = k[N2O5]
If the initial concentration of N2O5 is 0.80 M,the concentration after 5 half-lives is
A)0.11 M.
B)0.025 M.
C)0.032 M.
D)0.16 M.
E)0.050 M.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
40
What is the half-life of a reaction that has a rate constant of 280 s - 1?
A)194 s
B)3.6 ms
C)404 ms
D)Because the concentration of reactant is not given, the calculation cannot be performed.
E)2.5 ms
A)194 s
B)3.6 ms
C)404 ms
D)Because the concentration of reactant is not given, the calculation cannot be performed.
E)2.5 ms

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
41
The activation energy of a reaction is given by
A)+(slope of a plot of lnk vs 1/T)/ R.
B)(*slope of a plot of lnk vs 1/T)/ R.)
C)(-R / (slope of a plot of lnk vs 1/T).)
D)+(slope of a plot of lnk vs 1/T)* R.
E)(-0(slope of a plot of lnk vs 1/T)* R.)
A)+(slope of a plot of lnk vs 1/T)/ R.
B)(*slope of a plot of lnk vs 1/T)/ R.)
C)(-R / (slope of a plot of lnk vs 1/T).)
D)+(slope of a plot of lnk vs 1/T)* R.
E)(-0(slope of a plot of lnk vs 1/T)* R.)

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
42
The rate law for the following mechanism is ClO - (aq)+ H2O(l) ![<strong>The rate law for the following mechanism is ClO <sup>-</sup> (aq)+ H<sub>2</sub>O(l) HOCl(aq)+ OH <sup>-</sup> (aq) K,fast I <sup>-</sup> (aq)+ HOCl(aq) \rightarrow HOI(aq)+ Cl <sup>-</sup> 0(aq) K<sub>1</sub>,slow HOI(aq)+ OH <sup>-</sup> (aq) \rightarrow OI <sup>-</sup> (aq)+ H<sub>2</sub>O(l) K<sub>2</sub>,fast</strong> A)rate = k<sub>1</sub>[I <sup>-</sup> ][HOCl]. B)rate = k<sub>1</sub>K[ClO <sup>-</sup> ][I <sup>-</sup> ][OH <sup>-</sup> ]. C)rate = k<sub>1</sub>K[ClO <sup>-</sup> ][I <sup>-</sup> ][OH <sup>-</sup> ] <sup>-</sup> <sup>1</sup>.<sup> </sup> D)rate = k<sub>1</sub>k<sub>2</sub>K[ClO <sup>-</sup> ][I <sup>-</sup> ]. E)rate = k<sub>1</sub>K[ClO <sup>-</sup> ][I <sup>-</sup> ].](https://d2lvgg3v3hfg70.cloudfront.net/TB1039/11ea7e73_3895_2a23_9a0a_794bc5d79364_TB1039_11.jpg) HOCl(aq)+ OH - (aq)
HOCl(aq)+ OH - (aq)
K,fast
I - (aq)+ HOCl(aq) HOI(aq)+ Cl - 0(aq)
K1,slow
HOI(aq)+ OH - (aq) OI - (aq)+ H2O(l)
K2,fast
A)rate = k1[I - ][HOCl].
B)rate = k1K[ClO - ][I - ][OH - ].
C)rate = k1K[ClO - ][I - ][OH - ] - 1.
D)rate = k1k2K[ClO - ][I - ].
E)rate = k1K[ClO - ][I - ].
![<strong>The rate law for the following mechanism is ClO <sup>-</sup> (aq)+ H<sub>2</sub>O(l) HOCl(aq)+ OH <sup>-</sup> (aq) K,fast I <sup>-</sup> (aq)+ HOCl(aq) \rightarrow HOI(aq)+ Cl <sup>-</sup> 0(aq) K<sub>1</sub>,slow HOI(aq)+ OH <sup>-</sup> (aq) \rightarrow OI <sup>-</sup> (aq)+ H<sub>2</sub>O(l) K<sub>2</sub>,fast</strong> A)rate = k<sub>1</sub>[I <sup>-</sup> ][HOCl]. B)rate = k<sub>1</sub>K[ClO <sup>-</sup> ][I <sup>-</sup> ][OH <sup>-</sup> ]. C)rate = k<sub>1</sub>K[ClO <sup>-</sup> ][I <sup>-</sup> ][OH <sup>-</sup> ] <sup>-</sup> <sup>1</sup>.<sup> </sup> D)rate = k<sub>1</sub>k<sub>2</sub>K[ClO <sup>-</sup> ][I <sup>-</sup> ]. E)rate = k<sub>1</sub>K[ClO <sup>-</sup> ][I <sup>-</sup> ].](https://d2lvgg3v3hfg70.cloudfront.net/TB1039/11ea7e73_3895_2a23_9a0a_794bc5d79364_TB1039_11.jpg) HOCl(aq)+ OH - (aq)
HOCl(aq)+ OH - (aq)K,fast
I - (aq)+ HOCl(aq) HOI(aq)+ Cl - 0(aq)
K1,slow
HOI(aq)+ OH - (aq) OI - (aq)+ H2O(l)
K2,fast
A)rate = k1[I - ][HOCl].
B)rate = k1K[ClO - ][I - ][OH - ].
C)rate = k1K[ClO - ][I - ][OH - ] - 1.
D)rate = k1k2K[ClO - ][I - ].
E)rate = k1K[ClO - ][I - ].

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
43
A reaction has k = 8.39 M - 1.s - 1.How long does it take for the reactant concentration to drop from 0.0840 M to 0.0220 M?
A)5.42 s
B)2.00 s
C)1.42 s
D)8.39 s
E)4.00 s
A)5.42 s
B)2.00 s
C)1.42 s
D)8.39 s
E)4.00 s

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
44
The reaction [(CN)5CoOH2]2 - (aq)+ SCN - (aq) [(CN)5CoSCN]3 - + H2O(l)has the rate law,rate = k[(CN)5CoOH22 - ].Postulate a mechanism for this reaction.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
45
A second-order reaction has a rate constant of 1.25 M - 1.s - 1.If the initial reactant concentration is 1.0 M,calculate the time required for 90% reaction.
A)1.3 s
B)7.2 s
C)0.13 s
D)17 s
E)0.89 s
A)1.3 s
B)7.2 s
C)0.13 s
D)17 s
E)0.89 s

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
46
Consider the reaction for the dimerization of butadiene(g)at a certain temperature.
2C4H6(g) C8H12(g) rate = k[C4H6]2.
When the initial concentration of butadiene is 0.500 M,the time required for 80% dimerization is measured at 11.4 s.What is the rate constant for the dimerization?
2C4H6(g) C8H12(g) rate = k[C4H6]2.
When the initial concentration of butadiene is 0.500 M,the time required for 80% dimerization is measured at 11.4 s.What is the rate constant for the dimerization?

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
47
A reaction that has a very low activation energy
A)has a rate that is very sensitive to temperature.
B)has a rate that does not change much with temperature.
C)must be second order.
D)gives a curved Arrhenius plot.
E)must be first order.
A)has a rate that is very sensitive to temperature.
B)has a rate that does not change much with temperature.
C)must be second order.
D)gives a curved Arrhenius plot.
E)must be first order.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
48
The reaction 2NO(g)+ O2(g) 2NO2(g)has Hr = -114 kJ.mol - 1.A possible mechanism for this reaction is
2NO(g)b N2O2(g)
rapid equilibrium,K
N2O2(g)+ O2(g) 2NO2(g)
slow,k
Draw the reaction profile diagram (plot of energy vs reaction coordinate)for this reaction and label any intermediates and activated complexes.
2NO(g)b N2O2(g)
rapid equilibrium,K
N2O2(g)+ O2(g) 2NO2(g)
slow,k
Draw the reaction profile diagram (plot of energy vs reaction coordinate)for this reaction and label any intermediates and activated complexes.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
49
What is the half-life of a second order reaction?

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
50
Consider the dimerization reaction below: 2A A2
Rate = k[A]2
When the initial concentration of A is 2.0 M,it requires 30 min for 60% of A to react.Calculate the rate constant.
A)1.1 *10 - 3 M - 1.s - 1
B)3.2 *10 - 4 M - 1.s - 1
C)5.0 *10 - 4 M - 1.s - 1
D)1.9 *10 - 4 M - 1.s - 1
E)4.2 *10 - 4 M- 1.s - 1
Rate = k[A]2
When the initial concentration of A is 2.0 M,it requires 30 min for 60% of A to react.Calculate the rate constant.
A)1.1 *10 - 3 M - 1.s - 1
B)3.2 *10 - 4 M - 1.s - 1
C)5.0 *10 - 4 M - 1.s - 1
D)1.9 *10 - 4 M - 1.s - 1
E)4.2 *10 - 4 M- 1.s - 1

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
51
A certain reaction has a rate constant of 8.8 s - 1 at 298 K and 140 s - 1 at 323 K.What is the activation energy for this reaction?
A)38 kJ.mol - 1
B)89 kJ.mol - 1
C)120 kJ.mol - 1
D)23 kJ.mol - 1
E)1.2 kJ.mol - 1
A)38 kJ.mol - 1
B)89 kJ.mol - 1
C)120 kJ.mol - 1
D)23 kJ.mol - 1
E)1.2 kJ.mol - 1

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
52
The reaction profile for the reaction
[(CN)5CoOH2]2 - (aq)+ SCN - (aq) [(CN)5CoSCN]3 - + H2O(l)
is![The reaction profile for the reaction [(CN)<sub>5</sub>CoOH<sub>2</sub>]<sup>2</sup> <sup>-</sup> (aq)+ SCN <sup>-</sup> (aq) \rightarrow [(CN)<sub>5</sub>CoSCN]<sup>3</sup> <sup>-</sup> + H<sub>2</sub>O(l) is Identify the structure of B.What do A and C represent?](https://d2lvgg3v3hfg70.cloudfront.net/TB1039/11ea7e73_3895_0312_9a0a_ddffa353105d_TB1039_00.jpg) Identify the structure of B.What do A and C represent?
Identify the structure of B.What do A and C represent?
[(CN)5CoOH2]2 - (aq)+ SCN - (aq) [(CN)5CoSCN]3 - + H2O(l)
is
![The reaction profile for the reaction [(CN)<sub>5</sub>CoOH<sub>2</sub>]<sup>2</sup> <sup>-</sup> (aq)+ SCN <sup>-</sup> (aq) \rightarrow [(CN)<sub>5</sub>CoSCN]<sup>3</sup> <sup>-</sup> + H<sub>2</sub>O(l) is Identify the structure of B.What do A and C represent?](https://d2lvgg3v3hfg70.cloudfront.net/TB1039/11ea7e73_3895_0312_9a0a_ddffa353105d_TB1039_00.jpg) Identify the structure of B.What do A and C represent?
Identify the structure of B.What do A and C represent? 
Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
53
For the reaction HO(g)+ H2(g) H2O(g)+ H(g)
A plot of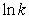 versus 1/T gives a straight line with a slope equal to -5.1 * 103 K.What is the activation energy for the reaction?
versus 1/T gives a straight line with a slope equal to -5.1 * 103 K.What is the activation energy for the reaction?
A)42 kJ.mol - 1
B)98 kJ.mol - 1
C)0.61 kJ.mol - 1
D)5.1 kJ.mol - 1
E)12 kJ.mol - 1
A plot of
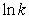 versus 1/T gives a straight line with a slope equal to -5.1 * 103 K.What is the activation energy for the reaction?
versus 1/T gives a straight line with a slope equal to -5.1 * 103 K.What is the activation energy for the reaction?A)42 kJ.mol - 1
B)98 kJ.mol - 1
C)0.61 kJ.mol - 1
D)5.1 kJ.mol - 1
E)12 kJ.mol - 1

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
54
Given: CH4(g)+ Cl2(g) CH3Cl(g)+ HCl(g)
The rate law for this elementary process is
A)rate = k[Cl2].
B)k[CH3Cl][HCl].
C)rate = k[CH4][Cl2].
D)rate = k[CH4].
E)rate = k[CH4]2.
The rate law for this elementary process is
A)rate = k[Cl2].
B)k[CH3Cl][HCl].
C)rate = k[CH4][Cl2].
D)rate = k[CH4].
E)rate = k[CH4]2.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
55
A catalyst facilitates a reaction by
A)increasing the activation energy for the reverse reaction.
B)lowering the activation energy of the reaction.
C)shifting the position of the equilibrium of the reaction.
D)decreasing the temperature at which the reaction will proceed spontaneously.
E)making the reaction more exothermic.
A)increasing the activation energy for the reverse reaction.
B)lowering the activation energy of the reaction.
C)shifting the position of the equilibrium of the reaction.
D)decreasing the temperature at which the reaction will proceed spontaneously.
E)making the reaction more exothermic.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
56
Consider the reaction NOBr(g) NO(g)+ ½Br2(g)
A plot of [NOBr] - 1 vs t gives a straight line with a slope of 2.00 M - 1.s - 1.The order of the reaction and the rate constant,respectively,are
A)second-order and 0.500 M - 1.s - 1.
B)first-order and 2.00 s - 1.
C)second-order and 2.00 M - 1.s - 1.
D)first-order and 0.241 s - 1.
E)second-order and 16.6 M - 1.s - 1.
A plot of [NOBr] - 1 vs t gives a straight line with a slope of 2.00 M - 1.s - 1.The order of the reaction and the rate constant,respectively,are
A)second-order and 0.500 M - 1.s - 1.
B)first-order and 2.00 s - 1.
C)second-order and 2.00 M - 1.s - 1.
D)first-order and 0.241 s - 1.
E)second-order and 16.6 M - 1.s - 1.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
57
For the reaction cyclobutane(g) 2ethylene(g)at 800 K,the half-life is 0.43 s.Calculate the time needed for the concentration of cyclobutane to fall to 1/64 of its initial value.
A)2.2 s
B)2.6 s
C)16 ms
D)0.38 s
E)0.43 s
A)2.2 s
B)2.6 s
C)16 ms
D)0.38 s
E)0.43 s

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
58
The reaction 2NO2(g) 2NO(g)+ O2(g)
Is postulated to occur via the mechanism
NO2(g)+ NO2(g) NO(g)+ NO3(g)
Slow
NO3(g) NO(g)+ O2(g)
Fast
An intermediate in this reaction is
A)NO2(g).
B)ON-NO3(g).
C)O2(g).
D)NO(g).
E)NO3(g).
Is postulated to occur via the mechanism
NO2(g)+ NO2(g) NO(g)+ NO3(g)
Slow
NO3(g) NO(g)+ O2(g)
Fast
An intermediate in this reaction is
A)NO2(g).
B)ON-NO3(g).
C)O2(g).
D)NO(g).
E)NO3(g).

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
59
An elementary process has an activation energy of 92 kJ/mol.If the enthalpy change for the reaction is -62 kJ/mol,what is the activation energy for the reverse reaction?
A)154 kJ/mol
B)62 kJ/mol
C)92 kJ/mol
D)30 kJ/mol
A)154 kJ/mol
B)62 kJ/mol
C)92 kJ/mol
D)30 kJ/mol

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
60
The rate law for the following mechanism is NO2(g)+ F2(g) NO2F(g)+ F(g)
K1,slow
F(g)+ NO2(g) NO2F(g)
K2,fast
A)rate = k2[NO2]2.
B)rate = k2[NO2][F].
C)rate = k1[NO2][F2].
D)rate = k1k2[NO2]2.
E)rate = k1[NO2F][F].
K1,slow
F(g)+ NO2(g) NO2F(g)
K2,fast
A)rate = k2[NO2]2.
B)rate = k2[NO2][F].
C)rate = k1[NO2][F2].
D)rate = k1k2[NO2]2.
E)rate = k1[NO2F][F].

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
61
Consider the following mechanism for the production of phosgene.
Cl2 Cl + Cl
Cl + CO COCl
COCl + Cl COCl2
The molecularity of the first and second elementary reactions is ____ and ____.
Cl2 Cl + Cl
Cl + CO COCl
COCl + Cl COCl2
The molecularity of the first and second elementary reactions is ____ and ____.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
62
For the elementary reaction A + 2B products,rate = k[A][B].True or false?

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
63
The fraction of molecules that collide with a kinetic energy equal to the activation energy for a reaction decreases rapidly with an increase in temperature.True or false?

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
64
A possible mechanism for the reaction A + B + D E + F is
A + B C
C
k1,k-1,fast
C + D E + F
k2
where C is a reactive intermediate present in very low concentrations.Calculate the steady state concentration of C in terms of the rate constants for the individual steps and the concentrations of the reactants.
A + B
 C
Ck1,k-1,fast
C + D E + F
k2
where C is a reactive intermediate present in very low concentrations.Calculate the steady state concentration of C in terms of the rate constants for the individual steps and the concentrations of the reactants.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
65
In the Michaelis-Menten mechanism of enzyme reaction,the Michaelis constant,KM,is
A)KM = k1/k2.
B)KM = k1'/k2.
C)KM = (k1' + k2)/k1.
D)KM = k1' + k2.
E)KM = k1/k1'.
A)KM = k1/k2.
B)KM = k1'/k2.
C)KM = (k1' + k2)/k1.
D)KM = k1' + k2.
E)KM = k1/k1'.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
66
The HBr synthesis is thought to involve the following reactions:
1.Br2 2Br.
2.Br. + H2 HBr + H.
3.H.+ Br2 HBr + Br.
4.2Br. Br2
5.2H. H2
"6.H. + Br. HBr
The chain propagation reactions in this mechanism are reactions"
A)6.
B)1, 2, 3, and 6.
C)2 and 3.
D)1, 2, and 3.
E)2, 3, and 6.
1.Br2 2Br.
2.Br. + H2 HBr + H.
3.H.+ Br2 HBr + Br.
4.2Br. Br2
5.2H. H2
"6.H. + Br. HBr
The chain propagation reactions in this mechanism are reactions"
A)6.
B)1, 2, 3, and 6.
C)2 and 3.
D)1, 2, and 3.
E)2, 3, and 6.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
67
The reduction of M3+ by Cr2+ was studied with [Cr2+] 100 times the concentration of Cr2+.When [Cr2+] = 0.0050 M,the rate was 2.5 s - 1.The rate constant for this reaction is
A)1.3 M - 1·s - 1
B)0.013 M - 1·s - 1
C)5.0 M - 1·s - 1
D)500 M - 1·s - 1
A)1.3 M - 1·s - 1
B)0.013 M - 1·s - 1
C)5.0 M - 1·s - 1
D)500 M - 1·s - 1

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
68
Given: A + B P rate = k[A][B] Which of the following is true?
A)k = ln 2/t1/2
B)ln [A]t = -kt + ln [A]o
C)[A]t = [A]o/(1 + kt[A]o)
D)1/[A]t = 1/kt
A)k = ln 2/t1/2
B)ln [A]t = -kt + ln [A]o
C)[A]t = [A]o/(1 + kt[A]o)
D)1/[A]t = 1/kt

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
69
The reaction between nitrogen dioxide and carbon monoxide is thought to occur by the mechanism 2NO2(g) NO3(g)+ NO(g)
K1,slow
NO3(g)+ CO(g) NO2(g)+ CO2(g)
K2,fast
The rate law for this mechanism is
A)rate = k1k2[NO2]2[CO].
B)rate = (k1/k2)[NO2]2[CO].
C)rate = k1[NO3][NO].
D)rate = k2[NO3][CO].
E)rate = k1[NO2]2.
K1,slow
NO3(g)+ CO(g) NO2(g)+ CO2(g)
K2,fast
The rate law for this mechanism is
A)rate = k1k2[NO2]2[CO].
B)rate = (k1/k2)[NO2]2[CO].
C)rate = k1[NO3][NO].
D)rate = k2[NO3][CO].
E)rate = k1[NO2]2.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
70
A possible mechanism for the reaction 2NO(g)+ O2(g) 2NO2(g)is: 2NO(g) ![<strong>A possible mechanism for the reaction 2NO(g)+ O<sub>2</sub>(g) \rightarrow 2NO<sub>2</sub>(g)is: 2NO(g) N<sub>2</sub>O<sub>2</sub>(g) K<sub>1</sub>,k<sub>1</sub>',fast N<sub>2</sub>O<sub>2</sub>(g)+ O<sub>2</sub>(g) \rightarrow 2NO<sub>2</sub>(g) K<sub>2</sub>,slow Application of the steady-state approximation gives</strong> A)[N<sub>2</sub>O<sub>2</sub>] = 0. B)k<sub>1</sub>[NO]<sup>2</sup> -k<sub>2</sub>[N<sub>2</sub>O<sub>2</sub>][O<sub>2</sub>] = 0. C)[N<sub>2</sub>O<sub>2</sub>] = (k<sub>1</sub>/k<sub>2</sub>)[NO]<sup>2</sup>.<sup> </sup> D)k<sub>1</sub>[NO]<sup>2</sup> -k<sub>1</sub>'[N<sub>2</sub>O<sub>2</sub>] -k<sub>2</sub>[N<sub>2</sub>O<sub>2</sub>][O<sub>2</sub>] = 0. E)[N<sub>2</sub>O<sub>2</sub>] = (k<sub>1</sub>/k<sub>1</sub>')[NO]<sup>2</sup>.<sup> </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB1039/11ea7e73_3895_7846_9a0a_03c36a62edeb_TB1039_11.jpg) N2O2(g)
N2O2(g)
K1,k1',fast
N2O2(g)+ O2(g) 2NO2(g)
K2,slow
Application of the steady-state approximation gives
A)[N2O2] = 0.
B)k1[NO]2 -k2[N2O2][O2] = 0.
C)[N2O2] = (k1/k2)[NO]2.
D)k1[NO]2 -k1'[N2O2] -k2[N2O2][O2] = 0.
E)[N2O2] = (k1/k1')[NO]2.
![<strong>A possible mechanism for the reaction 2NO(g)+ O<sub>2</sub>(g) \rightarrow 2NO<sub>2</sub>(g)is: 2NO(g) N<sub>2</sub>O<sub>2</sub>(g) K<sub>1</sub>,k<sub>1</sub>',fast N<sub>2</sub>O<sub>2</sub>(g)+ O<sub>2</sub>(g) \rightarrow 2NO<sub>2</sub>(g) K<sub>2</sub>,slow Application of the steady-state approximation gives</strong> A)[N<sub>2</sub>O<sub>2</sub>] = 0. B)k<sub>1</sub>[NO]<sup>2</sup> -k<sub>2</sub>[N<sub>2</sub>O<sub>2</sub>][O<sub>2</sub>] = 0. C)[N<sub>2</sub>O<sub>2</sub>] = (k<sub>1</sub>/k<sub>2</sub>)[NO]<sup>2</sup>.<sup> </sup> D)k<sub>1</sub>[NO]<sup>2</sup> -k<sub>1</sub>'[N<sub>2</sub>O<sub>2</sub>] -k<sub>2</sub>[N<sub>2</sub>O<sub>2</sub>][O<sub>2</sub>] = 0. E)[N<sub>2</sub>O<sub>2</sub>] = (k<sub>1</sub>/k<sub>1</sub>')[NO]<sup>2</sup>.<sup> </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB1039/11ea7e73_3895_7846_9a0a_03c36a62edeb_TB1039_11.jpg) N2O2(g)
N2O2(g)K1,k1',fast
N2O2(g)+ O2(g) 2NO2(g)
K2,slow
Application of the steady-state approximation gives
A)[N2O2] = 0.
B)k1[NO]2 -k2[N2O2][O2] = 0.
C)[N2O2] = (k1/k2)[NO]2.
D)k1[NO]2 -k1'[N2O2] -k2[N2O2][O2] = 0.
E)[N2O2] = (k1/k1')[NO]2.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
71
The reaction 2A + B D + E has the rate law,rate = a[A]2[B]/(b + c[A])where a,b,and c are constants.The following mechanism has been proposed for this reaction.
A + B![The reaction 2A + B \rightarrow D + E has the rate law,rate = a[A]<sup>2</sup>[B]/(b + c[A])where a,b,and c are constants.The following mechanism has been proposed for this reaction. A + B I k<sub>1</sub>,k<sub>-1</sub> I + A \rightarrow D + E k<sub>2</sub> I is an unstable intermediate present in minute concentrations.Show that this mechanism leads to the observed rate law and evaluate the constants a,b,and c in terms of the rate constants k<sub>1</sub>,k<sub>-1</sub>,and k<sub>2</sub>.](https://d2lvgg3v3hfg70.cloudfront.net/TB1039/11ea7e73_3895_ed79_9a0a_9bedfbf2baaa_TB1039_11.jpg) I
I
k1,k-1
I + A D + E
k2
I is an unstable intermediate present in minute concentrations.Show that this mechanism leads to the observed rate law and evaluate the constants a,b,and c in terms of the rate constants k1,k-1,and k2.
A + B
![The reaction 2A + B \rightarrow D + E has the rate law,rate = a[A]<sup>2</sup>[B]/(b + c[A])where a,b,and c are constants.The following mechanism has been proposed for this reaction. A + B I k<sub>1</sub>,k<sub>-1</sub> I + A \rightarrow D + E k<sub>2</sub> I is an unstable intermediate present in minute concentrations.Show that this mechanism leads to the observed rate law and evaluate the constants a,b,and c in terms of the rate constants k<sub>1</sub>,k<sub>-1</sub>,and k<sub>2</sub>.](https://d2lvgg3v3hfg70.cloudfront.net/TB1039/11ea7e73_3895_ed79_9a0a_9bedfbf2baaa_TB1039_11.jpg) I
Ik1,k-1
I + A D + E
k2
I is an unstable intermediate present in minute concentrations.Show that this mechanism leads to the observed rate law and evaluate the constants a,b,and c in terms of the rate constants k1,k-1,and k2.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
72
The reaction profile for the mechanism NO2(g)+ F2(g) NO2F(g)+ F(g)slow
F(g)+ NO2(g) NO2F(g)fast
Shows
A)two maxima, the first maximum being the higher.
B)two maxima, the second maximum being the higher.
C)one maximum for the second step.
D)two maxima, both the same height.
F(g)+ NO2(g) NO2F(g)fast
Shows
A)two maxima, the first maximum being the higher.
B)two maxima, the second maximum being the higher.
C)one maximum for the second step.
D)two maxima, both the same height.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
73
The rate law for the following mechanism is Cl2(g) ![<strong>The rate law for the following mechanism is Cl<sub>2</sub>(g) 2Cl(g) K<sub>1</sub>,fast Cl(g)+ CO(g) COCl(g) K<sub>2</sub>,fast COCl(g)+ Cl<sub>2</sub>(g) \rightarrow COCl<sub>2</sub>(g)+ Cl(g) K<sub>3</sub>,slow</strong> A)rate = k<sub>3</sub>K<sub>1</sub>0.5K<sub>2</sub>[CO][Cl<sub>2</sub>]<sup>1.5</sup>.<sup> </sup> B)rate = k<sub>3</sub>K<sub>1</sub>0.5K<sub>2</sub>[CO][Cl<sub>2</sub>]<sup>0.5</sup>.<sup> </sup> C)rate = k<sub>3</sub>K<sub>1</sub>K<sub>2</sub>[CO][Cl<sub>2</sub>]. D)rate = k<sub>3</sub>[COCl][Cl<sub>2</sub>]. E)rate = k<sub>3</sub>[COCl][Cl<sub>2</sub>]<sup>1.5</sup>.<sup> </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB1039/11ea7e73_3895_7844_9a0a_27e065b121cb_TB1039_11.jpg) 2Cl(g)
2Cl(g)
K1,fast
Cl(g)+ CO(g)![<strong>The rate law for the following mechanism is Cl<sub>2</sub>(g) 2Cl(g) K<sub>1</sub>,fast Cl(g)+ CO(g) COCl(g) K<sub>2</sub>,fast COCl(g)+ Cl<sub>2</sub>(g) \rightarrow COCl<sub>2</sub>(g)+ Cl(g) K<sub>3</sub>,slow</strong> A)rate = k<sub>3</sub>K<sub>1</sub>0.5K<sub>2</sub>[CO][Cl<sub>2</sub>]<sup>1.5</sup>.<sup> </sup> B)rate = k<sub>3</sub>K<sub>1</sub>0.5K<sub>2</sub>[CO][Cl<sub>2</sub>]<sup>0.5</sup>.<sup> </sup> C)rate = k<sub>3</sub>K<sub>1</sub>K<sub>2</sub>[CO][Cl<sub>2</sub>]. D)rate = k<sub>3</sub>[COCl][Cl<sub>2</sub>]. E)rate = k<sub>3</sub>[COCl][Cl<sub>2</sub>]<sup>1.5</sup>.<sup> </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB1039/11ea7e73_3895_7845_9a0a_2508d71ed89c_TB1039_11.jpg) COCl(g)
COCl(g)
K2,fast
COCl(g)+ Cl2(g) COCl2(g)+ Cl(g)
K3,slow
A)rate = k3K10.5K2[CO][Cl2]1.5.
B)rate = k3K10.5K2[CO][Cl2]0.5.
C)rate = k3K1K2[CO][Cl2].
D)rate = k3[COCl][Cl2].
E)rate = k3[COCl][Cl2]1.5.
![<strong>The rate law for the following mechanism is Cl<sub>2</sub>(g) 2Cl(g) K<sub>1</sub>,fast Cl(g)+ CO(g) COCl(g) K<sub>2</sub>,fast COCl(g)+ Cl<sub>2</sub>(g) \rightarrow COCl<sub>2</sub>(g)+ Cl(g) K<sub>3</sub>,slow</strong> A)rate = k<sub>3</sub>K<sub>1</sub>0.5K<sub>2</sub>[CO][Cl<sub>2</sub>]<sup>1.5</sup>.<sup> </sup> B)rate = k<sub>3</sub>K<sub>1</sub>0.5K<sub>2</sub>[CO][Cl<sub>2</sub>]<sup>0.5</sup>.<sup> </sup> C)rate = k<sub>3</sub>K<sub>1</sub>K<sub>2</sub>[CO][Cl<sub>2</sub>]. D)rate = k<sub>3</sub>[COCl][Cl<sub>2</sub>]. E)rate = k<sub>3</sub>[COCl][Cl<sub>2</sub>]<sup>1.5</sup>.<sup> </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB1039/11ea7e73_3895_7844_9a0a_27e065b121cb_TB1039_11.jpg) 2Cl(g)
2Cl(g)K1,fast
Cl(g)+ CO(g)
![<strong>The rate law for the following mechanism is Cl<sub>2</sub>(g) 2Cl(g) K<sub>1</sub>,fast Cl(g)+ CO(g) COCl(g) K<sub>2</sub>,fast COCl(g)+ Cl<sub>2</sub>(g) \rightarrow COCl<sub>2</sub>(g)+ Cl(g) K<sub>3</sub>,slow</strong> A)rate = k<sub>3</sub>K<sub>1</sub>0.5K<sub>2</sub>[CO][Cl<sub>2</sub>]<sup>1.5</sup>.<sup> </sup> B)rate = k<sub>3</sub>K<sub>1</sub>0.5K<sub>2</sub>[CO][Cl<sub>2</sub>]<sup>0.5</sup>.<sup> </sup> C)rate = k<sub>3</sub>K<sub>1</sub>K<sub>2</sub>[CO][Cl<sub>2</sub>]. D)rate = k<sub>3</sub>[COCl][Cl<sub>2</sub>]. E)rate = k<sub>3</sub>[COCl][Cl<sub>2</sub>]<sup>1.5</sup>.<sup> </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB1039/11ea7e73_3895_7845_9a0a_2508d71ed89c_TB1039_11.jpg) COCl(g)
COCl(g)K2,fast
COCl(g)+ Cl2(g) COCl2(g)+ Cl(g)
K3,slow
A)rate = k3K10.5K2[CO][Cl2]1.5.
B)rate = k3K10.5K2[CO][Cl2]0.5.
C)rate = k3K1K2[CO][Cl2].
D)rate = k3[COCl][Cl2].
E)rate = k3[COCl][Cl2]1.5.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
74
For the reaction A products,the following data were collected.  The half-life for this reaction is
The half-life for this reaction is
A)0.521 s
B)0.752 s
C)0.922 s
D)1.08 s
 The half-life for this reaction is
The half-life for this reaction isA)0.521 s
B)0.752 s
C)0.922 s
D)1.08 s

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
75
For a zero-order reaction,the rate constant has the same units as the rate of reaction.True or false?

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
76
Consider the reaction
A + B C + D rate = k[A]2
The time it takes for [A] to decrease from 1.0 to 0.50 M is the same as the time it takes for [A] to decrease from0.50 to 0.25 M.True or false?
A + B C + D rate = k[A]2
The time it takes for [A] to decrease from 1.0 to 0.50 M is the same as the time it takes for [A] to decrease from0.50 to 0.25 M.True or false?

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
77
The rate law for a reaction can be determined from the coefficients in the overall reaction.True or false?

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
78
The reaction 2NO(g)+ O2(g) 2NO2(g)has Hr = -114 kJ.mol - 1.A possible mechanism for this reaction is 2NO(g)  N2O2(g)
N2O2(g)
Rapid equilibrium,K
N2O2(g)+ O2(g) 2NO2(g)
Slow,k
If the activation energy for the reverse reaction is 225 kJ.mol - 1,what is the activation energy for the forward reaction?
A)Because an intermediate is involved, the forward activation energy cannot be calculated with the given information.
B)111 kJ.mol - 1
C)339 kJ.mol - 1
D)114 kJ.mol - 1
E)55.5 kJ.mol - 1
 N2O2(g)
N2O2(g)Rapid equilibrium,K
N2O2(g)+ O2(g) 2NO2(g)
Slow,k
If the activation energy for the reverse reaction is 225 kJ.mol - 1,what is the activation energy for the forward reaction?
A)Because an intermediate is involved, the forward activation energy cannot be calculated with the given information.
B)111 kJ.mol - 1
C)339 kJ.mol - 1
D)114 kJ.mol - 1
E)55.5 kJ.mol - 1

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
79
The HBr synthesis is thought to involve the following reactions:
1.Br2 2Br.
2.Br. + H2 HBr + H.
3.H. + Br2 HBr + Br.
4.2Br. Br2
5.2H. H2
"6.H. + Br. HBr
The chain termination reactions in this mechanism are reactions"
A)3, 4, and 5.
B)4 and 5.
C)4, 5, and 6.
D)6.
E)3 and 4.
1.Br2 2Br.
2.Br. + H2 HBr + H.
3.H. + Br2 HBr + Br.
4.2Br. Br2
5.2H. H2
"6.H. + Br. HBr
The chain termination reactions in this mechanism are reactions"
A)3, 4, and 5.
B)4 and 5.
C)4, 5, and 6.
D)6.
E)3 and 4.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
80
Consider the following mechanism for the destruction of ozone.
O3 + NO NO2 + O2
NO2 + O NO + O2
What is the catalyst in this reaction?
O3 + NO NO2 + O2
NO2 + O NO + O2
What is the catalyst in this reaction?

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck



