Deck 14: Electrochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/94
Play
Full screen (f)
Deck 14: Electrochemistry
1
If the standard potentials for the couples Cu2+/Cu,Ag+/Ag,and Fe2+/Fe are +0.34,+0.80,and -0.44 V,respectively,which is the strongest reducing agent?
A)Fe
B)Ag
C)Ag+
D)Cu
E)Fe2+
A)Fe
B)Ag
C)Ag+
D)Cu
E)Fe2+
Fe
2
The standard potential of the Cu2+/Cu electrode is +0.34 V and the standard potential of the cell Pb(s) Pb2+(aq)m Cu2+(aq) Cu(s)
Is +0.47 V.What is the standard potential of the Pb2+/Pb electrode?
A)(-0.26 V)
B)+0.81 V
C)(-0.81V)
D)(-0.13 V)
E)+0.13 V
Is +0.47 V.What is the standard potential of the Pb2+/Pb electrode?
A)(-0.26 V)
B)+0.81 V
C)(-0.81V)
D)(-0.13 V)
E)+0.13 V
(-0.13 V)
3
What is the proper cell diagram for the reaction 2AgCl(s)+ H2(g) 2Ag(s)+ 2H+(aq)+ 2Cl - (aq)
A)Pt Cl - (aq) H+(aq)m H2(g) AgCl(s) Ag(s)
B)Pt H2(g) H+(aq)m Cl - (aq) AgCl(s) Ag(s)
C)Ag(s) AgCl(s) Cl - (aq)m H+(aq) H2(g) Pt
D)Pt H2(g) H+(aq)m Cl - (aq) Ag(s) Pt
E)Ag(s) AgCl(s) H+(aq)m Cl - (aq) H2(g) Pt
A)Pt Cl - (aq) H+(aq)m H2(g) AgCl(s) Ag(s)
B)Pt H2(g) H+(aq)m Cl - (aq) AgCl(s) Ag(s)
C)Ag(s) AgCl(s) Cl - (aq)m H+(aq) H2(g) Pt
D)Pt H2(g) H+(aq)m Cl - (aq) Ag(s) Pt
E)Ag(s) AgCl(s) H+(aq)m Cl - (aq) H2(g) Pt
Pt H2(g) H+(aq)m Cl - (aq) AgCl(s) Ag(s)
4
When the Ag(s) AgCl(s) Cl - (aq)electrode acts as a cathode,the reaction is
A)Ag+(aq)+ e - Ag(s).
B)Ag(s)+ Cl - (aq) AgCl(s)+ e - .
C)Ag(s) Ag+(aq)+ e - .
D)2AgCl(s)+ 2e - 2Ag+(aq)+ Cl2(g).
E)AgCl(s)+ e - Ag(s)+ Cl - (aq).
A)Ag+(aq)+ e - Ag(s).
B)Ag(s)+ Cl - (aq) AgCl(s)+ e - .
C)Ag(s) Ag+(aq)+ e - .
D)2AgCl(s)+ 2e - 2Ag+(aq)+ Cl2(g).
E)AgCl(s)+ e - Ag(s)+ Cl - (aq).

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
5
How many electrons appear in the balanced half reaction: NO3 - (aq) NO(g),in acidic solution?
A)2
B)3
C)4
D)6
E)8
A)2
B)3
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
6
In the determination of iron in vitamins,Fe2+ is titrated with permanganate,MnO4 - ,in acidic solution.The products of the reaction are Fe3+ and Mn2+.In the balanced equation,the number of electrons transferred is
A)5
B)1
C)10
D)7
A)5
B)1
C)10
D)7

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
7
Given: Cr(OH)3(s) CrO42 - (aq),basic solution. How many electrons appear in the balanced half-reaction?
A)3
B)6
C)5
D)7
E)4
A)3
B)6
C)5
D)7
E)4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
8
In a working electrochemical cell (+ cell voltage),the electrons flow from the anode through the external circuit to the cathode.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
9
Given: N2H5+(aq) N2(g). How many electrons appear in the balanced half-reaction?
A)6
B)2
C)1
D)4
E)5
A)6
B)2
C)1
D)4
E)5

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
10
The standard potential of the Cu2+/Cu electrode is +0.34 V and the standard potential of the cell Ag(s) AgCl(s) Cl - (aq)m Cu2+(aq) Cu(s)
Is +0.12 V.What is the standard potential of the AgCl/Ag,Cl - electrode?
A)(-0.46 V)
B)(-0.22 V)
C)+0.24 V
D)+0.46 V
E)+0.22 V
Is +0.12 V.What is the standard potential of the AgCl/Ag,Cl - electrode?
A)(-0.46 V)
B)(-0.22 V)
C)+0.24 V
D)+0.46 V
E)+0.22 V

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
11
A cell that uses bromine to oxidize chloride ion under standard conditions at 298 K has a positive potential.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
12
True or false: A cell that uses bromine to oxidize hydrogen to H+ under standard conditions at 298 K has a negative potential?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
13
If the standard potentials for the couples Fe3+/Fe2+,MnO42 - ,H+/Mn2+,H2O,Zn2+/Zn,V3+/V2+,and Br2/Br - are +0.77,+1.51,-0.76,-0.26,and +1.09 V,respectively,which is the strongest oxidizing agent?
A)Zn2+
B)Fe3+
C)Mn2+
D)Br2
E)MnO4 -
A)Zn2+
B)Fe3+
C)Mn2+
D)Br2
E)MnO4 -

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
14
Given: Zn(s)+ OH - (aq)+ H2O(l)+ NO3 - (aq) Zn(OH)42 - (aq)+ NH3(g) If the coefficient of NO3 - in the balanced equation is 1,how many electrons are transferred in the reaction?
A)10
B)6
C)2
D)4
E)8
A)10
B)6
C)2
D)4
E)8

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
15
For the cell diagram Pt H2(g),H+(aq)m Cu2+(aq) Cu(s)
Which reaction occurs at the anode?
A)Cu(s) Cu2+(aq)+ 2e -
B)2H+(aq)+ 2e - H2(g)
C)2H+(aq)+ Cu(s) H2(g)+ Cu2+(aq)
D)Cu2+(aq)+ 2e - Cu(s)
E)H2(g) 2H+(aq)+ 2e -
Which reaction occurs at the anode?
A)Cu(s) Cu2+(aq)+ 2e -
B)2H+(aq)+ 2e - H2(g)
C)2H+(aq)+ Cu(s) H2(g)+ Cu2+(aq)
D)Cu2+(aq)+ 2e - Cu(s)
E)H2(g) 2H+(aq)+ 2e -

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
16
When a sample of an unknown metal is dropped into 1 M H+(aq)under standard conditions,bubbles are observed.The unknown metal could be silver.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
17
In a working electrochemical cell (+ cell voltage),the cations in the salt bridge move toward the cathode.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
18
When equilibrium is reached in an electrochemical cell,the voltage reaches its maximum value.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
19
Given: S2O42- (aq) SO32- (aq),basic solution. How many electrons appear in the balanced half-reaction?
A)3
B)6
C)1
D)4
E)2
A)3
B)6
C)1
D)4
E)2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
20
Given: PH3(g) P4(s),basic solution. How many electrons appear in the balanced half-reaction?
A)12
B)9
C)6
D)8
E)3
A)12
B)9
C)6
D)8
E)3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the following cell at standard conditions: Zn(s) Zn2+(aq)m Fe2+(aq) Fe(s)
Calculate the value of Gr for the reaction that occurs when current is drawn from this cell.
A)(-62 kJ.mol - 1 )
B)(-230 kJ.mol - 1 )
C)+62 kJ.mol - 1
D)+230 kJ.mol - 1
E)(-31 kJ.mol - 1 )
Calculate the value of Gr for the reaction that occurs when current is drawn from this cell.
A)(-62 kJ.mol - 1 )
B)(-230 kJ.mol - 1 )
C)+62 kJ.mol - 1
D)+230 kJ.mol - 1
E)(-31 kJ.mol - 1 )

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
22
If the standard potential for Tl3+(aq)/Tl+(aq)is 1.21 V and the standard potential for Tl+(aq)/Tl(s)is -0.34 V,calculate the standard potential for Tl3+(aq)+ 3e - Tl(s).
A)0.69 V
B)0.87 V
C)1.55 V
D)0.09 V
E)0.29 V
A)0.69 V
B)0.87 V
C)1.55 V
D)0.09 V
E)0.29 V

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is the strongest oxidizing agent?
A)O3
B)MnO4 -
C)Cr2O72 -
D)Cl2
A)O3
B)MnO4 -
C)Cr2O72 -
D)Cl2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
24
The standard potential of the cell Pb(s) PbSO4(s) SO42 - (aq)m Pb2+(aq) Pb(s)
Is +0.23 V at 25 C.Calculate the equilibrium constant for the reaction of 1 M Pb2+(aq)with 1M SO42 - (aq).
A)3.7 *1016
B)8.0 * 1017
C)6.0 * 107
D)1.7 * 10 - 8
E)7.7 *103
Is +0.23 V at 25 C.Calculate the equilibrium constant for the reaction of 1 M Pb2+(aq)with 1M SO42 - (aq).
A)3.7 *1016
B)8.0 * 1017
C)6.0 * 107
D)1.7 * 10 - 8
E)7.7 *103

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
25
Which species will oxidize Cr2+ but not Mn2+?
A)Pb4+
B)O3 in acidic medium
C)Zn2+
D)Fe2+
E)V3+
A)Pb4+
B)O3 in acidic medium
C)Zn2+
D)Fe2+
E)V3+

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the reaction: 2Ag+(aq)+ Cu(s) Cu2+(aq)+ 2Ag(s)
If the standard potentials of Ag+ and Cu2+ are +0.80 V and +0.34 V,respectively,calculate the value of E for the given reaction.
A)+1.48 V
B)(-1.26 V)
C)(-0.46 V)
D)+1.26 V
E)+0.46 V
If the standard potentials of Ag+ and Cu2+ are +0.80 V and +0.34 V,respectively,calculate the value of E for the given reaction.
A)+1.48 V
B)(-1.26 V)
C)(-0.46 V)
D)+1.26 V
E)+0.46 V

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
27
Calculate E for the following cell. Zn(s) Zn2+(aq)m Cl - aq AgCl(s) Ag(s)
A)+0.54 V
B)+1.20 V
C)(-1.20 V)
D)+0.98 V
E)(-0.54 V)
A)+0.54 V
B)+1.20 V
C)(-1.20 V)
D)+0.98 V
E)(-0.54 V)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
28
If the standard potential for Cu2+(aq)/Cu+(aq)is 0.15 V and the standard potential for Cu2+(aq)/Cu(s)is 0.34 V,calculate the standard potential for Cu+(aq)+ e - Cu(s).
A)+0.32 V
B)+0.64 V
C)+0.53 V
D)+0.83 V
E)+0.49 V
A)+0.32 V
B)+0.64 V
C)+0.53 V
D)+0.83 V
E)+0.49 V

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
29
Of the following five ions or molecules,which is the strongest reducing agent?
A)Fe +2
B)Cr2+
C)F-
D)H2
E)Co2+
A)Fe +2
B)Cr2+
C)F-
D)H2
E)Co2+

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
30
If the standard free energy change for combustion of 1 mole of CH4(g)is-818 kJ.mol - 1,calculate the standard voltage that could be obtained from a fuel cell using this reaction.
A)(-1.06 V)
B)+0.53 V
C)+4.24 V
D)+8.48 V
E)+1.06 V
A)(-1.06 V)
B)+0.53 V
C)+4.24 V
D)+8.48 V
E)+1.06 V

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
31
Which pair of metals will dissolve in nitric acid?
A)Pt, Ag
B)Pt, Au
C)Ag, Fe
D)Ag, Au
E)Pt, Fe
A)Pt, Ag
B)Pt, Au
C)Ag, Fe
D)Ag, Au
E)Pt, Fe

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
32
Which species will reduce Ag+ but not Fe2+?
A)Pt
B)Au
C)V
D)Cr
E)H2
A)Pt
B)Au
C)V
D)Cr
E)H2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
33
Which metal will dissolve in hydrochloric acid?
A)All the metals listed will dissolve.
B)Fe
C)Ag
D)Pt
E)Au
A)All the metals listed will dissolve.
B)Fe
C)Ag
D)Pt
E)Au

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
34
Which species will reduce Br2 but not V3+?
A)Ce
B)Zn
C)Cu
D)Cr2+
E)Al
A)Ce
B)Zn
C)Cu
D)Cr2+
E)Al

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
35
If the standard potential for Ti3+(aq)/Ti2+(aq)is -0.37 V and the standard potential for Ti2+(aq)/Ti(s)is -1.63 V,calculate the standard potential for Ti3+(aq)+ 3e - Ti(s).
A)(-1.19 V)
B)(-0.40 V)
C)(-2.00 V)
D)(-1.26 V)
E)(-1.21 V)
A)(-1.19 V)
B)(-0.40 V)
C)(-2.00 V)
D)(-1.26 V)
E)(-1.21 V)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following occurs when HCl(aq),Cu(s),and Fe(s)are mixed under standard conditions?
A)O2(g)is formed.
B)Cu(s)dissolves.
C)Cl2(g)is formed.
D)No reaction takes place.
E)Fe(s)dissolves.
A)O2(g)is formed.
B)Cu(s)dissolves.
C)Cl2(g)is formed.
D)No reaction takes place.
E)Fe(s)dissolves.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the following reaction: 2Cu+(aq) Cu(s)+ Cu2+(aq)
If the standard potentials of Cu2+ and Cu+ are +0.34 and +0.52 V,respectively,calculate the value of E for the given reaction.
A)+0.86 V
B)+0.70 V
C)+0.18 V
D)(-0.18 V)
E)(-0.70 V)
If the standard potentials of Cu2+ and Cu+ are +0.34 and +0.52 V,respectively,calculate the value of E for the given reaction.
A)+0.86 V
B)+0.70 V
C)+0.18 V
D)(-0.18 V)
E)(-0.70 V)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
38
The standard potential of the cell Ag(s) AgCl(s)m Cl - (aq) Cu2+(aq) Cu(s)
Is +0.12 V at 25 C.If the standard potential of the Cu2+/Cu couple is +0.34 V,calculate the standard potential of the AgCl/Ag,Cl - couple.
A)(-0.12 V)
B)(-0.46 V)
C)+0.46 V
D)(-0.22 V)
E)+0.22 V
Is +0.12 V at 25 C.If the standard potential of the Cu2+/Cu couple is +0.34 V,calculate the standard potential of the AgCl/Ag,Cl - couple.
A)(-0.12 V)
B)(-0.46 V)
C)+0.46 V
D)(-0.22 V)
E)+0.22 V

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
39
Given: Ag+(aq)+ e - Ag(s)
E° = 0.80 V
Fe3+(aq)+ e - Fe2+(aq)
E = 0.77 V
Cu2+(aq)+ 2e - Cu(s)
E = 0.34 V
Which is the strongest reducing agent?
A)Ag
B)Cu2+
C)Cu
D)Ag+
E)Fe2+
E° = 0.80 V
Fe3+(aq)+ e - Fe2+(aq)
E = 0.77 V
Cu2+(aq)+ 2e - Cu(s)
E = 0.34 V
Which is the strongest reducing agent?
A)Ag
B)Cu2+
C)Cu
D)Ag+
E)Fe2+

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following occurs when HNO3(aq),Cu(s),and Pt(s)are mixed under standard conditions?
A)Pt(s)dissolves.
B)No reaction takes place.
C)Cu(s)dissolves.
D)Pt(s)dissolves and H2(g)is formed.
E)Cu(s)dissolves and H2(g)is formed.
A)Pt(s)dissolves.
B)No reaction takes place.
C)Cu(s)dissolves.
D)Pt(s)dissolves and H2(g)is formed.
E)Cu(s)dissolves and H2(g)is formed.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
41
Consider the following cell: Zn(s Zn2+(aq,0.100 M)m Cl - (aq,? M) Cl2(g,0.500 atm) Pt
For this cell,E = 2.12 V and E = 2.27 V at 25 C.Calculate the Cl - (aq)concentration in the cathode compartment.
A)1.2*10 - 1 M
B)4.3 *10 - 5 M
C)2.9 *10 - 3 M
D)1.5 *10 - 3 M
E)6.5 *10 - 3 M
For this cell,E = 2.12 V and E = 2.27 V at 25 C.Calculate the Cl - (aq)concentration in the cathode compartment.
A)1.2*10 - 1 M
B)4.3 *10 - 5 M
C)2.9 *10 - 3 M
D)1.5 *10 - 3 M
E)6.5 *10 - 3 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
42
Use the following to answer questions 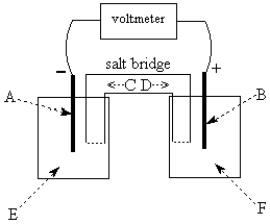
The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.The electrode B could be inert platinum metal or lead.True or false?

The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.The electrode B could be inert platinum metal or lead.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
43
Use the following to answer questions 
The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.The electrode B could be inert platinum metal or zinc.True or false?

The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.The electrode B could be inert platinum metal or zinc.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
44
The standard voltage of the cell Pt H2(g) H+(aq)m Cl - (aq) AgCl(s) Ag(s)
Is +0.22 V at 25 C.Calculate the equilibrium constant for the reaction below.
2AgCl(s)+ H2(g) 2Ag(s)+ 2H+(aq)+ 2Cl - (aq)
A)3.7
B)7.4
C)5.2 * 103
D)1.7 * 103
E)2.7 *107
Is +0.22 V at 25 C.Calculate the equilibrium constant for the reaction below.
2AgCl(s)+ H2(g) 2Ag(s)+ 2H+(aq)+ 2Cl - (aq)
A)3.7
B)7.4
C)5.2 * 103
D)1.7 * 103
E)2.7 *107

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
45
Use the following diagram of a cell to answer questions 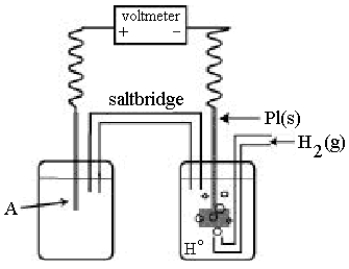
-In the cell shown above,A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is-0.76 V,what is the equation for the cell reaction?
A)Zn2+(aq)+ H2(g) Zn(s)+ 2H+(aq)
B)Zn(s)+ 2H+(aq) Zn2+(aq)+ H2(g)

-In the cell shown above,A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is-0.76 V,what is the equation for the cell reaction?
A)Zn2+(aq)+ H2(g) Zn(s)+ 2H+(aq)
B)Zn(s)+ 2H+(aq) Zn2+(aq)+ H2(g)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
46
The standard voltage of the cell Ag(s) AgBr(s) Br - (aq)m Ag+(aq) Ag(s)
Is +0.73 V at 25 C.Calculate the equilibrium constant for the cell reaction.
A)5.1 * 1014
B)2.0 *10 - 15
C)2.2 * 1012
D)4.6 *10 - 13
E)3.9 * 10 - 29
Is +0.73 V at 25 C.Calculate the equilibrium constant for the cell reaction.
A)5.1 * 1014
B)2.0 *10 - 15
C)2.2 * 1012
D)4.6 *10 - 13
E)3.9 * 10 - 29

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
47
Calculate E for the half-reaction below. 2H+(aq,1.00 *10 - 5 M)+ 2e - H2(g,1.00 atm)
A)0 V
B)+0.592 V
C)(-0.592 V)
D)(-0.296 V)
E)+0.296 V
A)0 V
B)+0.592 V
C)(-0.592 V)
D)(-0.296 V)
E)+0.296 V

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
48
What is the value of E for the following reaction?
2H+(aq,1.00 M)+ 2e - H2(g,1.00 atm)
2H+(aq,1.00 M)+ 2e - H2(g,1.00 atm)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
49
If E for the following cell is 0.36 V at 25 C Pb(s) PbSO4(s) SO42 - (aq,0.60 M)m H+(aq,0.70 M) H2(g,192.5 kPa) Pt
How is the Nernst equation for the cell properly expressed at this temperature?
A)E = 0.36 -0.01285ln[1.90/{(0.70)2(0.60)}]
B)E = 0.36 -0.02569ln[192.5/{(0.70)2(0.60)}]
C)E = 0.36 + 0.01285ln[192.5/{(0.70)2(0.60)}]
D)E = 0.36 + 0.01285ln[1.90/{(0.70)2(0.60)}]
E)E = 0.36 -0.01285ln[1.90/{(0.70)(0.60)}]
How is the Nernst equation for the cell properly expressed at this temperature?
A)E = 0.36 -0.01285ln[1.90/{(0.70)2(0.60)}]
B)E = 0.36 -0.02569ln[192.5/{(0.70)2(0.60)}]
C)E = 0.36 + 0.01285ln[192.5/{(0.70)2(0.60)}]
D)E = 0.36 + 0.01285ln[1.90/{(0.70)2(0.60)}]
E)E = 0.36 -0.01285ln[1.90/{(0.70)(0.60)}]

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
50
Consider the following cell: Pt H2(g,1 atm) H+(aq,? M)m Ag+(aq,1.0 M) Ag(s)
If the voltage of this cell is 1.04 V at 25 C and the standard potential of the Ag+/Ag couple is +0.80 V,calculate the hydrogen ion concentration in the anode compartment.
A)4.6 *10 - 10 M
B)8.8 *10 - 5 M
C)9.4 * 10 - 3 M
D)1.0 M
E)3.7 *10 - 8 M
If the voltage of this cell is 1.04 V at 25 C and the standard potential of the Ag+/Ag couple is +0.80 V,calculate the hydrogen ion concentration in the anode compartment.
A)4.6 *10 - 10 M
B)8.8 *10 - 5 M
C)9.4 * 10 - 3 M
D)1.0 M
E)3.7 *10 - 8 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the following cell:
Zn(s) Zn2+(aq,0.200 M)m H+(aq,?) H2(g,1.00 atm) Pt
If E = +0.66 V and E = +0.76 V at 25 C,calculate the concentration of H+ in the cathode cell compartment.
A)2.1 *10-2 M
B)4.0 *101 M
C)8.4 *10-5 M
D)9.2*10-3 M
E)4.0 *10-3 M
Zn(s) Zn2+(aq,0.200 M)m H+(aq,?) H2(g,1.00 atm) Pt
If E = +0.66 V and E = +0.76 V at 25 C,calculate the concentration of H+ in the cathode cell compartment.
A)2.1 *10-2 M
B)4.0 *10
C)8.4 *10-5 M
D)9.2*10-3 M
E)4.0 *10-3 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
52
The equilibrium constant for the reaction 2Hg(l)+ 2Cl - (aq)+ Ni2+(aq) Ni(s)+ Hg2Cl2(s)
Is 5.6 *10 - 20 at 25 C.Calculate the value of E for a cell utilizing this reaction.
A)+0.57 V
B)(-0.25 V)
C)+1.14 V
D)(-1.14 V)
E)(-0.57 V)
Is 5.6 *10 - 20 at 25 C.Calculate the value of E for a cell utilizing this reaction.
A)+0.57 V
B)(-0.25 V)
C)+1.14 V
D)(-1.14 V)
E)(-0.57 V)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
53
Consider the following cell:
Pt Fe2+(aq,0.50 M),Fe3+(aq,0.30 M)m Fe3+(aq,0.035 M),Fe2+(aq,0.010) Pt
The standard potential for the Fe3+/Fe2+ couple is +0.77 V.Calculate the cell voltage at 25 C.
Pt Fe2+(aq,0.50 M),Fe3+(aq,0.30 M)m Fe3+(aq,0.035 M),Fe2+(aq,0.010) Pt
The standard potential for the Fe3+/Fe2+ couple is +0.77 V.Calculate the cell voltage at 25 C.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
54
Use the following to answer questions 
-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Identify A and write the half-reaction that occurs in that compartment.Does the size of the electrode A increase or decrease during operation of the cell? What is the voltmeter reading?

-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Identify A and write the half-reaction that occurs in that compartment.Does the size of the electrode A increase or decrease during operation of the cell? What is the voltmeter reading?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
55
If E for the disproportionation of Cu+(aq)to Cu2+(aq)and Cu(s)is +0.18 V at 25 C,calculate the equilibrium constant for the reaction.
A)1.2 * 106
B)3.9 * 1074
C)2.5 * 1014
D)35.7
E)3.3 * 1012
A)1.2 * 106
B)3.9 * 1074
C)2.5 * 1014
D)35.7
E)3.3 * 1012

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
56
Use the following diagram of a cell to answer questions 
-In the cell shown above,A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is -0.76 V,which half-reaction occurs in the left-hand cell compartment?
A)Zn2+(aq)+ 2e - Zn(s)
B)Zn(s) Zn2+(aq)+ 2e -

-In the cell shown above,A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is -0.76 V,which half-reaction occurs in the left-hand cell compartment?
A)Zn2+(aq)+ 2e - Zn(s)
B)Zn(s) Zn2+(aq)+ 2e -

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
57
The standard potential of the cell Pb(s) PbSO4(s) SO42 - (aq)m Pb2+(aq) Pb(s)
Is +0.23 V at 25 C.Calculate the Ksp of PbSO4.
A)1.3 *10 - 18
B)1.7*10 - 8
C)1.3 * 10 - 4
D)2.7* *10 - 17
E)6.0 * 107
Is +0.23 V at 25 C.Calculate the Ksp of PbSO4.
A)1.3 *10 - 18
B)1.7*10 - 8
C)1.3 * 10 - 4
D)2.7* *10 - 17
E)6.0 * 107

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
58
Consider the following cell: Ag(s) Ag+(aq,0.100 M)mAg+(aq,0.100 M) Ag(s)
What is the voltage of this cell?
A)0
B)+0.0592 V
C)+0.0296 V
D)+0.80 V
What is the voltage of this cell?
A)0
B)+0.0592 V
C)+0.0296 V
D)+0.80 V

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
59
The standard voltage of the cell Ag(s) AgBr(s) Br - (aq)m Ag+(aq) Ag(s)
Is +0.73 V at 25 C.Calculate the Ksp for AgBr.
A)3.9 *10 - 29
B)2.2 * 1012
C)2.0*10 - 15
D)5.1 * 1014
E)4.6 *10 - 13
Is +0.73 V at 25 C.Calculate the Ksp for AgBr.
A)3.9 *10 - 29
B)2.2 * 1012
C)2.0*10 - 15
D)5.1 * 1014
E)4.6 *10 - 13

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
60
Use the following to answer questions 
-The galvanic cell shown above uses the half-cells Mg2+/Mg and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Identify A and write the half-reaction that occurs in that compartment.Does the size of the electrode A increase or decrease during operation of the cell? What is the voltmeter reading?

-The galvanic cell shown above uses the half-cells Mg2+/Mg and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Identify A and write the half-reaction that occurs in that compartment.Does the size of the electrode A increase or decrease during operation of the cell? What is the voltmeter reading?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
61
When KI(aq)is electrolyzed at a concentration of 1 M,the product at the anode is I2.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
62
The products of the electrolysis of CuSO4(aq)are
A)H2(g)and H2SO3(aq).
B)H2SO3(aq)and O2(g).
C)Cu(s)and H2SO3(aq).
D)Cu(s)and O2(g).
E)H2(g)and O2(g).
A)H2(g)and H2SO3(aq).
B)H2SO3(aq)and O2(g).
C)Cu(s)and H2SO3(aq).
D)Cu(s)and O2(g).
E)H2(g)and O2(g).

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
63
How many seconds are required to produce 4.99 mg of chromium metal from an acidic solution of potassium dichromate,using a current of 0.234 A?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
64
For the reduction of Cu2+ by Zn, Go = -212 kJ/mol and Eo = +1.10 V.If the coefficients in the chemical equation for this reaction are multiplied by 2, Go = -424 kJ/mol.This means Eo = +2.20 V.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
65
How many moles of Cl2(g)are produced by the electrolysis of concentrated sodium chloride if 2.00 A are passed through the solution for 4.00 hours? The equation for this process,the "chloralkali" process,is 2NaCl(aq)+ 2H2O(l) 2NaOH(aq)+ H2(g)+ Cl2(g)
A)0.0745 mol
B)0.149 mol
C)0.447 mol
D)0.00248 mol
E)0.298 mol
A)0.0745 mol
B)0.149 mol
C)0.447 mol
D)0.00248 mol
E)0.298 mol

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
66
What current is required to produce 91.6 g of chromium meta from chromium(VI)oxide in 12.4 hours?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
67
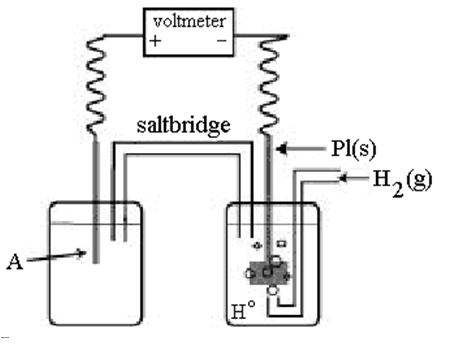
-Using the cell shown above,A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE).When the voltmeter reading is -0.80 V,which half-reaction occurs in the left-hand cell compartment?
A)Ag(s) Ag+(aq)+ e -
B)Ag+(aq)+ e - Ag(s)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
68
Of the following metals,which metal would be suitable to provide an iron bridge with cathodic protection from corrosion?
A)Ni
B)Cu
C)Pb
D)Sn
E)None of the metals listed is suitable.
A)Ni
B)Cu
C)Pb
D)Sn
E)None of the metals listed is suitable.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
69
Sodium is produced by electrolysis of molten sodium chloride.What are the products at the anode and cathode,respectively?
A)Na(l)and O2(g)
B)Cl - (aq)and Na2O(l)
C)Cl2(g)and Na2O(l)
D)O2(g)and Na(l)
E)Cl2(g)and Na(l)
A)Na(l)and O2(g)
B)Cl - (aq)and Na2O(l)
C)Cl2(g)and Na2O(l)
D)O2(g)and Na(l)
E)Cl2(g)and Na(l)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
70
When a lead-acid battery discharges,sulfuric acid is produced.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
71
In the cell shown above,A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is +0.80 V,which electrode is negative?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
72
How many moles of O2(g)are produced by electrolysis of Na2SO4(aq)if 0.120 A is passed through the solution for 65.0 min?
A)0.001 21 mol
B)0.000 080 8 mol
C)0.002 42 mol
D)0.004 85 mol
E)0.000 020 2 mol
A)0.001 21 mol
B)0.000 080 8 mol
C)0.002 42 mol
D)0.004 85 mol
E)0.000 020 2 mol

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
73
The half-reaction that occurs at cathode when 1 M AgNO3(aq)is electrolyzed is __________________.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
74
In order to convert hydrazine,N2H4,to nitric acid,
A)an oxidizing agent is required.
B)a reducing agent is required.
A)an oxidizing agent is required.
B)a reducing agent is required.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
75

-In the cell shown above,A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is +0.80 V,what is the equation for the cell reaction?
A)Ag(s)+ H+(aq) Ag+(aq)+ ½H2(g)
B)Ag+(aq)+ ½H2(g) Ag(s)+ H+(aq)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
76
In the Daniell cell,the anode is zinc metal and the cathode is copper metal.When the cell operates,the anode gets smaller and the cathode gets larger.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
77
Galvanized iron is protected from corrosion because zinc reduces any Fe2+ formed.True or false?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
78
How long will it take to deposit 0.00235 moles of gold by electrolysis of KAuCl4(aq)using a current of 0.214 amperes?
A)17.7 min
B)26.5 min
C)70.7 min
D)106 min
E)53.0 min
A)17.7 min
B)26.5 min
C)70.7 min
D)106 min
E)53.0 min

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
79

-In the cell shown above,A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is -0.76 V,which electrode is negative?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
80
If 8686 C of charge is passed through molten magnesium chloride,calculate the number of moles of Mg(l)produced.
A)0.0225 mol
B)0.0450 mol
C)2.00 mol
D)0.0110 mol
E)0.0900 mol
A)0.0225 mol
B)0.0450 mol
C)2.00 mol
D)0.0110 mol
E)0.0900 mol

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck



