Deck 10: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/96
Play
Full screen (f)
Deck 10: Nuclear Chemistry
1
A particle corresponds to
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)
2
When 131I emits a particle, what nuclide is produced?
A) 130I
B) 127Sb
C) 131Xe
D) 131Te
E) 130Te
A) 130I
B) 127Sb
C) 131Xe
D) 131Te
E) 130Te
131Xe
3
The nuclear equation for the disintegration of U-238 produces Th-234 .What other nuclide is produced?
A) 4He
B) neutron
C) positron
D) 3He
E)
A) 4He
B) neutron
C) positron
D) 3He
E)
4He
4
Bombarding 54Fe with a neutron results in emission of a proton and formation of
A) 55Fe.
B) 54Cr.
C) 54Co.
D) 54Mn.
E) 49Ti.
A) 55Fe.
B) 54Cr.
C) 54Co.
D) 54Mn.
E) 49Ti.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
5
When an particle is emitted, the atomic number
A) increases by 4.
B) decreases by 1.
C) decreases by 2.
D) decreases by 4.
E) increases by 2.
A) increases by 4.
B) decreases by 1.
C) decreases by 2.
D) decreases by 4.
E) increases by 2.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
6
A nuclide undergoes decay and forms 231Th .What is the nuclide?
A) 235Pu
B) 235U
C) 235Th
D) 237U
E) 231Pa
A) 235Pu
B) 235U
C) 235Th
D) 237U
E) 231Pa

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
7
What nuclide is formed when 234Th undergoes decay?
A) 232Pu
B) 234Pa
C) 234U
D) 233Th
E) 230Rn
A) 232Pu
B) 234Pa
C) 234U
D) 233Th
E) 230Rn

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
8
What nuclide undergoes decay to produce the daughter nuclide polonium-218 (Z = 84)?
A) radon-222
B) radium-226
C) polonium-222
D) polonium-214
E) radon-226
A) radon-222
B) radium-226
C) polonium-222
D) polonium-214
E) radon-226

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
9
A nuclide undergoes proton emission to form 52Fe .What is the nuclide?
A) 52Mn
B) 53Fe
C) 56Ni
D) 52Co
E) 53Co
A) 52Mn
B) 53Fe
C) 56Ni
D) 52Co
E) 53Co

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
10
How many nucleons does an particle contain?
A) 4
B) 0
C) 2
D) 8
E) 6
A) 4
B) 0
C) 2
D) 8
E) 6

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
11
If U-238 undergoes emission, what other nuclide is produced?
A) Np-236
B) Ac-236
C) Th-234
D) U-234
E) Pa-234
A) Np-236
B) Ac-236
C) Th-234
D) U-234
E) Pa-234

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
12
When a positron encounters an electron,both are annihilated and converted to energy.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
13
How many nucleons does boron-11 contain?
A) 6
B) 10
C) 5
D) 11
E) 16
A) 6
B) 10
C) 5
D) 11
E) 16

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
14
A nuclide undergoes positron emission to form 22Ne .What is the nuclide?
A) 23Mg
B) 18O
C) 22Na
D) 22F
E) 23Na
A) 23Mg
B) 18O
C) 22Na
D) 22F
E) 23Na

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
15
The nuclear equation for the disintegration of In-116 produces Sn-116 and
A) a positron.
B) helium-4.
C) a neutron.
D) gamma rays.
E) an electron.
A) a positron.
B) helium-4.
C) a neutron.
D) gamma rays.
E) an electron.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
16
Heavy elements are more likely to give off radiation.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
17
Heavy elements are more likely to give off radiation.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
18
An particle corresponds to
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
19
What nuclide is formed when 90Sr undergoes decay?
A) 86Kr
B) 90Y
C) 94Zr
D) 89Sr
E) 90Rb
A) 86Kr
B) 90Y
C) 94Zr
D) 89Sr
E) 90Rb

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
20
When an particle is emitted, the mass number
A) decreases by 4.
B) increases by 2.
C) decreases by 2.
D) decreases by 1.
E) increases by 4.
A) decreases by 4.
B) increases by 2.
C) decreases by 2.
D) decreases by 1.
E) increases by 4.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is most likely to undergo decay?
A) 222Rn
B) 11Li
C) 235U
D) 114Cs
E) 226Ra
A) 222Rn
B) 11Li
C) 235U
D) 114Cs
E) 226Ra

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
22
All of the following are stable except
A) 15O.
B) 20Ne.
C) 14N.
D) 12C.
E) 16O.
A) 15O.
B) 20Ne.
C) 14N.
D) 12C.
E) 16O.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
23
Boron neutron capture therapy is used to destroy tumors.In this reaction,10B absorbs a slow neutron to produce 11B*,which produces 7Li and __________ plus high-energy photons.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following nuclei is stable?
A) 120Sn
B) 213Fr
C) 38Cl
D) 24Na
E) 15O
A) 120Sn
B) 213Fr
C) 38Cl
D) 24Na
E) 15O

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
25
What is the element produced when 83Sr undergoes positron emission?
A) Kr
B) Br
C) Rb
D) Zr
E) Y
A) Kr
B) Br
C) Rb
D) Zr
E) Y

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
26
Elements with an odd number of protons and neutrons
A) are usually stable.
B) are usually unstable.
C) never undergo emission.
D) always have a proton-to-neutron ratio of about 1.
E) have a magic number of protons or neutrons.
A) are usually stable.
B) are usually unstable.
C) never undergo emission.
D) always have a proton-to-neutron ratio of about 1.
E) have a magic number of protons or neutrons.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
27
All the following are predicted to be unstable except
A) Ca-40
B) N-16
C) Na-24
D) O-15
A) Ca-40
B) N-16
C) Na-24
D) O-15

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
28
What type of particle is emitted in the transformation
201Pt 201Au
A) particle
B) positron
C) No particle is emitted because electron capture occurs.
D) particle
E) particle
201Pt 201Au
A) particle
B) positron
C) No particle is emitted because electron capture occurs.
D) particle
E) particle

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
29
What is the identity of the product,P,in the reaction58Fe + 2 P +

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
30
If a nuclide lies above the band of stability, it should decay by
A) Such nuclides do not decay.
B) -particle emission.
C) positron emission.
D) neutron emission.
E) -particle emission.
A) Such nuclides do not decay.
B) -particle emission.
C) positron emission.
D) neutron emission.
E) -particle emission.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is most penetrating?
A)
B)
C) +
D) p
E) n
A)
B)
C) +
D) p
E) n

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is expected to be stable?
A) 208Pb
B) 7Be
C) 122Sb
D) 87Kr
E) 24Na
A) 208Pb
B) 7Be
C) 122Sb
D) 87Kr
E) 24Na

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
33
Boron neutron capture therapy is used to destroy tumors.In this reaction,10B absorbs a slow neutron to produce 11B*,which produces particles and __________ plus high-energy photons.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
34
Nuclides that lie below the band of stability decay by positron emission,electron capture,or proton emission.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is most likely to undergo decay?
A) 24Na
B) 47K
C) 14C
D) 232Th
E) 3H
A) 24Na
B) 47K
C) 14C
D) 232Th
E) 3H

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
36
What is the element produced when 44Ti undergoes electron capture?
A) Ar
B) K
C) Sc
D) V
E) Ca
A) Ar
B) K
C) Sc
D) V
E) Ca

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
37
What type of particle is captured and what type is emitted in the nucleosynthesis
Fe 54Mn
A) neutron capture and proton emission
B) neutron capture and particle emission
C) only neutron capture occurs
D) neutron capture and positron emission occurs
E) electron capture and particle emission occurs
Fe 54Mn
A) neutron capture and proton emission
B) neutron capture and particle emission
C) only neutron capture occurs
D) neutron capture and positron emission occurs
E) electron capture and particle emission occurs

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
38
What process occurs when 222Rn forms the daughter nuclide 218Po?
A) Electron capture
B) emission
C) Positron emission
D) Neutron emission
E) emission
A) Electron capture
B) emission
C) Positron emission
D) Neutron emission
E) emission

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
39
Technetium-99m,a "metastable" nuclide of Tc is produced by the reaction_____ 99mTc +
What is the starting nuclide?
What is the starting nuclide?

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
40
What nuclide is formed when 211Po undergoes decay?
A) 207Pb
B) 206Ra
C) 206Pb
D) 208Rn
A) 207Pb
B) 206Ra
C) 206Pb
D) 208Rn

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
41
What is the time needed for the activity of a radium-226 source to change from 1.5 Ci to 0.50 Ci? (The half-life of radium-226 is 1.60 * 103 y.)
A) 5.33 * 102 y
B) 1.60 * 103 y
C) 1.10 * 103 y
D) 9.36 * 102 y
E) 2.54 * 103 y
A) 5.33 * 102 y
B) 1.60 * 103 y
C) 1.10 * 103 y
D) 9.36 * 102 y
E) 2.54 * 103 y

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
42
Calculate the time required for the activity of a 9.0-mCi sodium-25 source to decay to 7.0-mCi (the half-life of sodium-25 is 60.0 s).
A) 22 s
B) 44 s
C) 0.029 s
D) 19 s
E) 9.4 s
A) 22 s
B) 44 s
C) 0.029 s
D) 19 s
E) 9.4 s

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
43
Calculate the time required for the activity of a 9.0-mCi cobalt-60 source to decay to 8.5-mCi .The half-life of cobalt-60 is 5.26 years.
A) 4.6 months
B) 2.3 months
C) 5.2 months
D) 10 months
E) 0.090 months
A) 4.6 months
B) 2.3 months
C) 5.2 months
D) 10 months
E) 0.090 months

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
44
A sample of a dish from an archaeological dig gave 18 500 disintegrations per gram of carbon per day .A 1.00-g sample of carbon from a modern source gave 22 080 disintegrations per day.If the decay constant of carbon-14 is 1.2 * 10-4 y-1,What is the age of the dish?
A) 1500 y
B) 8300 y
C) 68 000 y
D) 640 y
E) 30 000 y
A) 1500 y
B) 8300 y
C) 68 000 y
D) 640 y
E) 30 000 y

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
45
Measurement by carbon-14 dating assumes that the proportion of C-14 in the atmosphere now and when the sample was alive is the same.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
46
The decay constant for strontium-90 is 0.0247 y-1 .How many grams of strontium-90 remain if 10.0 g decay for exactly 60 years?
A) 0.23 g
B) 0.33 g
C) 1.48 g
D) 2.27 g
E) 7.71 g
A) 0.23 g
B) 0.33 g
C) 1.48 g
D) 2.27 g
E) 7.71 g

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
47
The binding energy of a nucleus is
A) the energy evolved when a nucleus breaks up into fundamental particles.
B) the energy released when protons and neutrons come together to form a nucleus.
C) related to the number of electrons in a nucleus.
D) the energy required to eject one neutron from the nucleus.
E) related to the mass lost in a chemical reaction.
A) the energy evolved when a nucleus breaks up into fundamental particles.
B) the energy released when protons and neutrons come together to form a nucleus.
C) related to the number of electrons in a nucleus.
D) the energy required to eject one neutron from the nucleus.
E) related to the mass lost in a chemical reaction.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
48
A 9.9-g sample of iodine-131 is stored for exactly 3 weeks .If the decay constant is 0.0861 day-1,What mass of the isotope remains?
A) 0.15 g
B) 7.6 g
C) 1.6 g
D) 2.6 g
E) 5.5 g
A) 0.15 g
B) 7.6 g
C) 1.6 g
D) 2.6 g
E) 5.5 g

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
49
The outcomes of positron emission and electron capture are the same: the atomic number is reduced by 1.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
50
Cobalt-60, used in the radiation treatment of cancer,Is produced by absorption of 2 neutrons and emission of a particle.What is the starting nuclide?
A) 58Mn
B) 58Ni
C) 58Fe
D) 59Co
E) 59Fe
A) 58Mn
B) 58Ni
C) 58Fe
D) 59Co
E) 59Fe

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
51
Calculate the nuclear binding energy of 1 mol lithium-7.
A) 6.31 *10-12 kJ
B) 1.14 * 1015 kJ
C) 6.31 * 10-15 kJ
D) 1.14 * 1018 kJ
E) 3.80 * 109 kJ
A) 6.31 *10-12 kJ
B) 1.14 * 1015 kJ
C) 6.31 * 10-15 kJ
D) 1.14 * 1018 kJ
E) 3.80 * 109 kJ

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
52
The nuclear binding energy for calcium-40 is the energy released when
A) calcium-39 and 1 neutron form calcium-40.
B) 20 protons and 20 neutrons form calcium-40.
C) 20 protons and 20 electrons form calcium-20.
D) 40 neutrons form calcium-40.
E) 40 protons form calcium-40.
A) calcium-39 and 1 neutron form calcium-40.
B) 20 protons and 20 neutrons form calcium-40.
C) 20 protons and 20 electrons form calcium-20.
D) 40 neutrons form calcium-40.
E) 40 protons form calcium-40.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
53
Radioactive decay is a
A) first-order process.
B) second-order process.
C) nonspontaneous process.
D) temperature-dependent process.
E) zero-order process.
A) first-order process.
B) second-order process.
C) nonspontaneous process.
D) temperature-dependent process.
E) zero-order process.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
54
In the 238U decay series, there are 8 particles and 6 particles lost starting with emission from 238U.What is the final product?
A) 207Pb
B) 210Po
C) 210Bi
D) 208Pb
E) 206Pb
A) 207Pb
B) 210Po
C) 210Bi
D) 208Pb
E) 206Pb

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
55
Use the law of radioactive decay to determine the activity of a 2.0- g sample of Tc-99, t½ = 2.2 * 105 y.
A) 1.0 Ci
B) 1.0 * 10-6 Ci
C) 1.5 Ci
D) 3.3 *10-8 Ci
E) 3.3 * 10-2 Ci
A) 1.0 Ci
B) 1.0 * 10-6 Ci
C) 1.5 Ci
D) 3.3 *10-8 Ci
E) 3.3 * 10-2 Ci

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
56
The nuclear binding energy for lithium-7 is the energy released in the nuclear reaction
A) 31H + 7n 7Li.
B) 6Li + n 7Li.
C) 31H + 4 7Li.
D) 31H + 4n 7Li.
E) 71H 7Li.
A) 31H + 7n 7Li.
B) 6Li + n 7Li.
C) 31H + 4 7Li.
D) 31H + 4n 7Li.
E) 71H 7Li.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
57
When 214Pb decays as part of the 238U decay series, there are 2 particles and 4 particles lost starting with emission from 214Pb.What is the final product?
A) 207Pb
B) 206Pb
C) 210Bi
D) 210Po
E) 210Pb
A) 207Pb
B) 206Pb
C) 210Bi
D) 210Po
E) 210Pb

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
58
The nuclear binding energy for iron-56 is the energy released in the nuclear reaction
A) 261H + 30 56Fe
B) 561H 56Fe
C) 55Fe + n 56Fe
D) 261H + 56n 56Fe
E) 261H + 30n 56Fe
A) 261H + 30 56Fe
B) 561H 56Fe
C) 55Fe + n 56Fe
D) 261H + 56n 56Fe
E) 261H + 30n 56Fe

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
59
The half-life of plutonium-239 is 24 100 years .Calculate the percent of a plutonium sample left after 100 years.
A) 99.7%
B) 4.15%
C) 90.0%
D) 50.0%
E) 96.4%
A) 99.7%
B) 4.15%
C) 90.0%
D) 50.0%
E) 96.4%

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
60
The half-life of strontium-90 is 28.1 years .Calculate the percent of a strontium sample left after 100 years.
A) 76%
B) 0.34%
C) 63%
D) 82%
E) 8.5%
A) 76%
B) 0.34%
C) 63%
D) 82%
E) 8.5%

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
61
Nuclides that lie below the band of stability are proton rich and can form more stable nuclei by _______________,___________________,and __________________.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
62
What type of reaction is it when a neutron becomes a proton,and the result is a nucleus that changes from Z to Z+1?

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
63
Calculate the energy change when one 235U nucleus undergoes the fission reaction
235U + n 142Ba + 92Kr + 2n
The masses needed are 235U,235.04 u; 142Ba,141.92 u; 92Kr,91.92 u; n,1.0087 u.
A) -3.2 * 10-28 J
B) +1.8 * 10-10 J
C) -1.7 * 10-10 J
D) +2.9 * 10-11 J
E) -2.8 * 10-11 J
235U + n 142Ba + 92Kr + 2n
The masses needed are 235U,235.04 u; 142Ba,141.92 u; 92Kr,91.92 u; n,1.0087 u.
A) -3.2 * 10-28 J
B) +1.8 * 10-10 J
C) -1.7 * 10-10 J
D) +2.9 * 10-11 J
E) -2.8 * 10-11 J

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
64
In the following equations,fill in the missing species.
(a)
41Ca + ___ 41K
(b)
15O 15N + ___
(a)
41Ca + ___ 41K
(b)
15O 15N + ___

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
65
What type of particle is emitted in the reaction below? Give an example of this process. 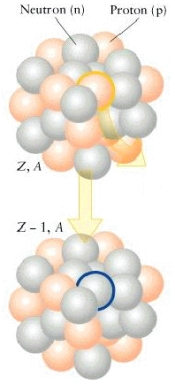


Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
66
Calculate the energy change for the synthesis of 1 mol 60Co
58Fe + 2n 60Co +
A) 2.53 * 10-15 kJ
B) 1.52 * 109 kJ
C) 2.53 * 10-12 kJ
D) The calculation cannot be done without the mass of .
E) 8.43 * 10-24 kJ
58Fe + 2n 60Co +
A) 2.53 * 10-15 kJ
B) 1.52 * 109 kJ
C) 2.53 * 10-12 kJ
D) The calculation cannot be done without the mass of .
E) 8.43 * 10-24 kJ

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following nuclei is likely to capture an electron? What is the daughter nuclide produced?
A) 9Li and 5He
B) 41Ca and 41K
C) 246Am and 246Pu
D) 80Ge and 80Ga
E) 24Mg and 5He
A) 9Li and 5He
B) 41Ca and 41K
C) 246Am and 246Pu
D) 80Ge and 80Ga
E) 24Mg and 5He

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
68
The equation
6D 24He + 21H + 2n
Represents
A) the binding energy of He.
B) fusion.
C) electron capture.
D) fission.
E) the decay of a proton.
6D 24He + 21H + 2n
Represents
A) the binding energy of He.
B) fusion.
C) electron capture.
D) fission.
E) the decay of a proton.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
69
The curie is defined as the radioactive output of 1.00 g of radium-226.Calculate this value in disintegrations per second (the half-life of radium-226 is 1600 years).

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following nuclei is most likely to emit a positron?
A) 246Am
B) 59Ni
C) 118Sn
D) 33Cl
E) 20Ne
A) 246Am
B) 59Ni
C) 118Sn
D) 33Cl
E) 20Ne

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
71
For the fusion of two deuterium atoms to produce helium-4,the difference in mass between products and reactants is -0.0256 u.What is E per mole of helium?

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
72
Complete the nuclear reaction
97Mo + 2H _____ + 2n
97Mo + 2H _____ + 2n

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
73
Why does the band of stability curve upward at high atomic number?

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
74
An particle is an example of a doubly magic nucleus.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
75
What nuclide is formed when 24Na undergoes decay?
A) 28S
B) 24Ne
C) 28P
D) 24Mg
A) 28S
B) 24Ne
C) 28P
D) 24Mg

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
76
In the following equations, fill in the missing species.
(a)
___ + 7Li
(b)
9Li ___ +
(a)
___ + 7Li
(b)
9Li ___ +

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
77
A 1.00-g sample of carbon from a modern source gave 15.3 disintegrations per minute.A sample of carbon from a presumably old source gave 920 disintegrations per hour.What is the age of the "old" sample of carbon? The half-life of carbon-14 is 5.73 * 103 yr.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
78
For the thermonuclear reaction
D + T 4He + n
the difference in mass between products and reactants is -0.0189 u.What is E per mole of helium?
D + T 4He + n
the difference in mass between products and reactants is -0.0189 u.What is E per mole of helium?

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
79
A sample of carbon from the Lascaux cave in France contained 12% of the original fraction carbon-14.Estimate the age of this sample (the half-life of carbon-14 is 5.73 * 103 year).

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck
80
One becquerel is equal to _________________________ and one curie is equal to _________________________.

Unlock Deck
Unlock for access to all 96 flashcards in this deck.
Unlock Deck
k this deck



