Deck 18: Heat and the First Law of Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/67
Play
Full screen (f)
Deck 18: Heat and the First Law of Thermodynamics
1
For a class demonstration,your physics instructor pours 1.00 kg of steam at 100 C over 5.00 kg of ice at 0.00 C,allows the system to reach equilibrium and then he is going to measure the temperature of the system.While the system used in the class demonstration reaches equilibrium,you are given the latent heats of ice and steam and the specific heat of water to be,respectively: 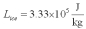 ;
; 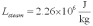 ;
; 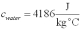 and you are asked to calculate the final equilibrium temperature of the system.What value did you find?
and you are asked to calculate the final equilibrium temperature of the system.What value did you find?
A)22.5 C
B)40.4 C
C)64.3 C
D)80.3 C
E)95.8 C
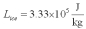 ;
; 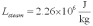 ;
; 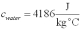 and you are asked to calculate the final equilibrium temperature of the system.What value did you find?
and you are asked to calculate the final equilibrium temperature of the system.What value did you find?A)22.5 C
B)40.4 C
C)64.3 C
D)80.3 C
E)95.8 C
40.4 C
2
A 2.0-kg piece of steel with a temperature of 70 C is submerged in 1.0 kg of water at 15 C.At what temperature does the water/metal system reach equilibrium? The specific heat of steel is 0.107 cal/(g * K).
A)17 C
B)25 C
C)38 C
D)42 C
A)17 C
B)25 C
C)38 C
D)42 C
25 C
3
A copper sheet of area 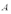 is bonded to an unknown metal sheet of area
is bonded to an unknown metal sheet of area 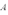 .The thickness of the unknown metal is half that of the copper.The outside surface of the copper sheet is held at a temperature of 80 C and the unknown metal sheet at 40 C.If the temperature of the copper-unknown metal interface is 60 C,what is the thermal conductivity of the unknown metal?
.The thickness of the unknown metal is half that of the copper.The outside surface of the copper sheet is held at a temperature of 80 C and the unknown metal sheet at 40 C.If the temperature of the copper-unknown metal interface is 60 C,what is the thermal conductivity of the unknown metal?
A)386 W/(m*K)
B)193 W/(m*K)
C)258 W/(m*K)
D)772 W/(m*K)
E)220 W/(m*K)
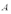 is bonded to an unknown metal sheet of area
is bonded to an unknown metal sheet of area 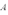 .The thickness of the unknown metal is half that of the copper.The outside surface of the copper sheet is held at a temperature of 80 C and the unknown metal sheet at 40 C.If the temperature of the copper-unknown metal interface is 60 C,what is the thermal conductivity of the unknown metal?
.The thickness of the unknown metal is half that of the copper.The outside surface of the copper sheet is held at a temperature of 80 C and the unknown metal sheet at 40 C.If the temperature of the copper-unknown metal interface is 60 C,what is the thermal conductivity of the unknown metal?A)386 W/(m*K)
B)193 W/(m*K)
C)258 W/(m*K)
D)772 W/(m*K)
E)220 W/(m*K)
193 W/(m*K)
4
How many joules of energy need to be added to a 1-cm3 block of lead to increase its temperature from room temperature (20 C)to 37 C?
A)275 J
B)193 J
C)7.8 J
D)17 J
E)25 J
A)275 J
B)193 J
C)7.8 J
D)17 J
E)25 J

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
5
A gas has an initial volume of 1.00 m3.It is expanded to five times its original volume through a quasi-static process for which P = a V 4,with a = 3.00 N/m14.How much work is done by the expanding gas?
A)0.975 kJ
B)1.28 kJ
C)1.87 kJ
D)2.24 kJ
E)3.40 kJ
A)0.975 kJ
B)1.28 kJ
C)1.87 kJ
D)2.24 kJ
E)3.40 kJ

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
6
A copper sheet of area 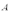 is bonded to an aluminum sheet of area
is bonded to an aluminum sheet of area 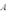 and thickness 1.00 mm.The outside surface of the copper sheet is held at a temperature of 80 C and the aluminum sheet at 40 C.The temperature of the copper-aluminum interface is 52 C.What is the thickness of the copper sheet?
and thickness 1.00 mm.The outside surface of the copper sheet is held at a temperature of 80 C and the aluminum sheet at 40 C.The temperature of the copper-aluminum interface is 52 C.What is the thickness of the copper sheet?
A)2.8 mm
B)3.6 mm
C)4.1 mm
D)5.2 mm
E)6.3 mm
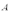 is bonded to an aluminum sheet of area
is bonded to an aluminum sheet of area 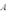 and thickness 1.00 mm.The outside surface of the copper sheet is held at a temperature of 80 C and the aluminum sheet at 40 C.The temperature of the copper-aluminum interface is 52 C.What is the thickness of the copper sheet?
and thickness 1.00 mm.The outside surface of the copper sheet is held at a temperature of 80 C and the aluminum sheet at 40 C.The temperature of the copper-aluminum interface is 52 C.What is the thickness of the copper sheet?A)2.8 mm
B)3.6 mm
C)4.1 mm
D)5.2 mm
E)6.3 mm

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
7
When the volume of a thermodynamic system decreases,
A)the volume of the environment decreases.
B)the graph of pressure versus volume is always a straight line.
C)the work is never positive.
D)the temperature measured in Kelvin is never positive.
A)the volume of the environment decreases.
B)the graph of pressure versus volume is always a straight line.
C)the work is never positive.
D)the temperature measured in Kelvin is never positive.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
8
On which large surface should you place a hot pot if you want to cool off the pot as quickly as possible?
A)a smooth glass surface
B)a smooth steel surface
C)a rough steel surface
D)a smooth wood surface
E)a rough wood surface
A)a smooth glass surface
B)a smooth steel surface
C)a rough steel surface
D)a smooth wood surface
E)a rough wood surface

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
9
A gas has an initial volume of 3.00 m3.It is expanded to four times its original volume through a quasi-static process for which P = a V 2,with a = 6.00 N/m8.How much work is done by the expanding gas?
A)0.975 kJ
B)1.28 kJ
C)1.87 kJ
D)2.24 kJ
E)3.40 kJ
A)0.975 kJ
B)1.28 kJ
C)1.87 kJ
D)2.24 kJ
E)3.40 kJ

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
10
A 2.00-g lead bullet is shot with a speed of 250 m/s into a wooden wall.Assuming that all of its kinetic energy is absorbed as heat by the bullet,what is the temperature increase of the bullet?
A)96.8 C
B)101 C
C)242 C
D)358 C
E)357 C
A)96.8 C
B)101 C
C)242 C
D)358 C
E)357 C

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
11
The pressure of a dilute gas as a function of volume is given as p(V)= C/V,where the pressure is measured in Pascal,the volume in cubic meters and C is a constant measured in Joules.How much work is done by such a gas when it expands from an initial volume Vi to a final volume Vf?
A)C
B)C (Vf /Vi)
C)C ln(Vf /Vi)
D)C ln(Vf -Vi)
A)C
B)C (Vf /Vi)
C)C ln(Vf /Vi)
D)C ln(Vf -Vi)

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
12
A 2.00-g lead bullet is shot into a wooden wall.As a result,the temperature of the bullet increases by 212 C.Assuming that all of its kinetic energy is absorbed as heat by the bullet,what was the speed of the bullet just prior to striking the wall?
A)250 m/s
B)202 m/s
C)280 m/s
D)234 m/s
E)271 m/s
A)250 m/s
B)202 m/s
C)280 m/s
D)234 m/s
E)271 m/s

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
13
A copper sheet of area 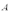 is bonded to an aluminum sheet of area
is bonded to an aluminum sheet of area 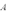 .Both sheets have the same thickness.The outside surface of the copper sheet is held at a temperature of 80 C and the aluminum sheet at 40 C.What is the temperature of the copper-aluminum interface?
.Both sheets have the same thickness.The outside surface of the copper sheet is held at a temperature of 80 C and the aluminum sheet at 40 C.What is the temperature of the copper-aluminum interface?
A)48 C
B)65 C
C)70 C
D)52 C
E)60 C
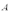 is bonded to an aluminum sheet of area
is bonded to an aluminum sheet of area 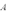 .Both sheets have the same thickness.The outside surface of the copper sheet is held at a temperature of 80 C and the aluminum sheet at 40 C.What is the temperature of the copper-aluminum interface?
.Both sheets have the same thickness.The outside surface of the copper sheet is held at a temperature of 80 C and the aluminum sheet at 40 C.What is the temperature of the copper-aluminum interface?A)48 C
B)65 C
C)70 C
D)52 C
E)60 C

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
14
A 2.00-g lead bullet is shot with a speed of 300 m/s into a wooden wall.Assume that all of its kinetic energy is absorbed by the bullet.Which statement is true? (The melting point of lead is 327 C.)
A)The bullet will melt.
B)The bullet will not melt.
C)The bullet will have a kinetic energy equal to 9.0 * 104 J.
D)The bullet will have a kinetic energy equal to 9.0 * 103 J.
A)The bullet will melt.
B)The bullet will not melt.
C)The bullet will have a kinetic energy equal to 9.0 * 104 J.
D)The bullet will have a kinetic energy equal to 9.0 * 103 J.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
15
A lead bullet is shot with a speed of 250 m/s into a wooden wall.As a result,the temperature of the bullet increases by 358 C.Assuming that all of its kinetic energy is absorbed as heat by the bullet,what is the mass of the bullet?
A)1.5 g
B)2.0 g
C)2.5 g
D)2.8 g
E)The bullet's mass cannot be determined from the given information
A)1.5 g
B)2.0 g
C)2.5 g
D)2.8 g
E)The bullet's mass cannot be determined from the given information

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
16
Heat is the same as
A)temperature.
B)thermodynamic work.
C)change in internal energy.
D)None are correct.
A)temperature.
B)thermodynamic work.
C)change in internal energy.
D)None are correct.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
17
It takes 2.7 * 103 calories of energy to heat a small piece of aluminum from 100 F to 120 F.What is the mass of the piece of aluminum?
A)1.1 kg
B)2.5 kg
C)3.6 kg
D)4.2 kg
A)1.1 kg
B)2.5 kg
C)3.6 kg
D)4.2 kg

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
18
For an adiabatic process,
A)the work done by the system is always zero.
B)no heat ever flows between the system and its environment.
C)the change of the system's internal energy is always zero.
D)None are correct.
A)the work done by the system is always zero.
B)no heat ever flows between the system and its environment.
C)the change of the system's internal energy is always zero.
D)None are correct.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
19
A gas enclosed in a cylinder by means of a piston that can move without friction is heated so that the increase in the internal energy of the gas is 1500 J.Assuming that the volume of the gas is constant,the heat that entered the gas is
A)1500 J.
B)-1500 J.
C)0.
D)It is impossible to determine.
E)None are correct.
A)1500 J.
B)-1500 J.
C)0.
D)It is impossible to determine.
E)None are correct.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
20
5.00 kg of some liquid at 10.0 C is mixed with 1.00 kg of the same liquid at 40.0 C.What is the final equilibrium temperature? Ignore any heat flow with the containers and/or surroundings.
A)12.0 C
B)15.0 C
C)18.0 C
D)25.0 C
A)12.0 C
B)15.0 C
C)18.0 C
D)25.0 C

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following processes are adiabatic?
A)a truck skidding to a stop
B)an ice cube melting on a sidewalk
C)a radiator heating a room
D)a balloon inflating
E)None are correct.
A)a truck skidding to a stop
B)an ice cube melting on a sidewalk
C)a radiator heating a room
D)a balloon inflating
E)None are correct.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
22
A 0.25-liter bottle of apple juice contains 120 food calories.How many of these bottles of apple juice would be required to match the energy content in a liter of gasoline,where 87 octane gasoline contains 32 MJ per liter?
A)4 bottles
B)16 bottles
C)32 bottles
D)64 bottles
E)128 bottles
A)4 bottles
B)16 bottles
C)32 bottles
D)64 bottles
E)128 bottles

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
23
During his honeymoon trip in 1847 near the falls at Chamonix in the French Alps,Joule took along a thermometer to measure the temperature at the top and bottom of the falls 120 m high.He assumed the total kinetic energy of the water at the bottom becomes internal energy of the water.An estimate of the rise in temperature of the water of specific heat 4190 J/kg K at the bottom is
A)28 C.
B)12 C.
C)7 C.
D)1.5 C.
E)0.28 C.
A)28 C.
B)12 C.
C)7 C.
D)1.5 C.
E)0.28 C.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
24
Assume that 10 g of steam is added to 100 g of water initially at 19 C.The water is inside an aluminum cup of mass 35 g.The cup is inside a perfectly insulating calorimetric container that prevents heat flow from the outside environment.Find the final temperature of the water after equilibrium is reached. Specific heat of water: 4.19 kJ/(kg * K)or 1.00 cal/(g * K)
Specific heat of aluminum: 922 J/(kg * K)or 0.22 cal/(g * K)
Latent heat of fusion for water: 334 kJ/kg or 79.7 cal/g
Latent heat of vaporization for water: 2260 kJ/kg or 539 cal/g
A)25 C
B)26 C
C)31 C
D)72 C
E)76 C
Specific heat of aluminum: 922 J/(kg * K)or 0.22 cal/(g * K)
Latent heat of fusion for water: 334 kJ/kg or 79.7 cal/g
Latent heat of vaporization for water: 2260 kJ/kg or 539 cal/g
A)25 C
B)26 C
C)31 C
D)72 C
E)76 C

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
25
The work done per cycle by a gas following the pv trajectory of figure shown is 90 J.Determine the maximum pressure,Pmax. 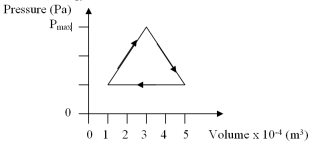
A)300 kPa
B)3 kPa
C)30 kPa
D)600 kPa
E)6 kPa

A)300 kPa
B)3 kPa
C)30 kPa
D)600 kPa
E)6 kPa

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
26
Seven liters of a fluid with a specific heat of 2.93 kJ/(kg * K)or 0.700 cal/(g * K)at 20 C are mixed with three liters of the same fluid at 32 C.Suppose that the fluid is insulated so that no energy can flow into it or out of it.What is the final temperature of the fluid? You do not know the density of the fluid,but you do know that the density is constant over this temperature range.
A)11 C
B)24 C
C)25 C
D)31 C
E)The mass density of the material is needed.
A)11 C
B)24 C
C)25 C
D)31 C
E)The mass density of the material is needed.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
27
How different would the rate of heat transfer from a hot reservoir to a cold reservoir be if one uses a 10-cm glass rod instead of a 10-m long aluminum rod having identical cross sectional areas?
A)There would be no change.
B)2.75 times faster
C)2.75 times slower
D)275 times faster
E)275 times slower
A)There would be no change.
B)2.75 times faster
C)2.75 times slower
D)275 times faster
E)275 times slower

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
28
In which process is there no work done on a gas?
A)isothermal
B)isochoric
C)Isobaric
D)None are correct.
A)isothermal
B)isochoric
C)Isobaric
D)None are correct.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
29
How much work is done per cycle by a gas following the pv trajectory of figure shown? 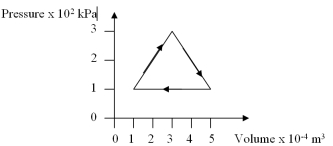
A)40 J
B)0 J
C)5 J
D)3 J
E)80 J

A)40 J
B)0 J
C)5 J
D)3 J
E)80 J

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
30
How much work is done to expand a 10-cm diameter cylindrical chamber at 1 atm from a length of 10 cm to a length of 20 cm?
A)234.5 J
B)317.3 J
C)79.3 J
D)175.2 J
E)286.5 J
A)234.5 J
B)317.3 J
C)79.3 J
D)175.2 J
E)286.5 J

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
31
A 25-g piece of aluminum at 85 C is dropped in 1 liter of water at 10 C which is in an insulated beaker.Assuming that there is negligible heat loss to the surrounding,determine the equilibrium temperature of the system.
A)25.0ºC
B)10.4ºC
C)75.0ºC
D)47.5ºC
E)83.6ºC
A)25.0ºC
B)10.4ºC
C)75.0ºC
D)47.5ºC
E)83.6ºC

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
32
A 10-cm copper cube sits on a heat source with a 10-cm cube of ice sitting on top of the copper cube.What is the minimum required temperature of the heat source if all of the ice is to melt in 154 seconds? The density of ice is 917 kg/m3 and the thermal conductivity of copper is 386 W/(m·K).
A)79 C
B)109 C
C)100 C
D)54 C
E)65 C
A)79 C
B)109 C
C)100 C
D)54 C
E)65 C

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
33
If a person drops a penny (m = 2.5 g)off of the top of the Statue of Liberty (h = 46.84 m)and it lands in a water barrel of water (159 kg).Find the change in temperature of the water,assuming all of the kinetic energy of the penny goes into heating the water.Ignore the effects of air resistance on the penny and the heat capacity of the penny.The specific heat of water is 4.19 kJ/(kg * K)or 1.00 cal/(g * K).
A)0.18 K
B)1.7 K
C)0.74 mK
D)7.2 mK
E)None are correct.
A)0.18 K
B)1.7 K
C)0.74 mK
D)7.2 mK
E)None are correct.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
34
A 10-cm copper cube sits on a 100 C heat source with a 10-cm cube of ice sitting on top of the copper cube.How long does it take the ice to melt? The density of ice is 0.917 kg/m3 and the thermal conductivity of copper is 386 W/(m·K).
A)383 s
B)412 s
C)213 s
D)79 s
E)154 s
A)383 s
B)412 s
C)213 s
D)79 s
E)154 s

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
35
A 12-fluid oz.(355 g)soft drink label states that it has 150 dietary calories.The drink is initially at 10 C before it consumed it then reaches body temperature of 37 C.Find the net energy gained by the person who drank it (Hint: You can treat the soft drink to be identical to water in terms of its heat capacity,c = 4.19 kJ/(kg K)= 1.00 cal/(g K).)
A)10 Cal
B)126 Cal
C)140 Cal
D)143 Cal
E)150 Cal
A)10 Cal
B)126 Cal
C)140 Cal
D)143 Cal
E)150 Cal

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following processes takes more energy?
A)heating 1 kg water from 110 C to 200 C
B)melting a 500-g block of ice at 0 C
C)evaporating 100 g of water at 100 C by boiling
D)bringing 1 kg of water from 90 C to 100 C
E)All of the choices use the same amount of energy.
A)heating 1 kg water from 110 C to 200 C
B)melting a 500-g block of ice at 0 C
C)evaporating 100 g of water at 100 C by boiling
D)bringing 1 kg of water from 90 C to 100 C
E)All of the choices use the same amount of energy.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
37
It takes 59,0.25-liter bottles of orange juice contains to match the energy content in a liter of gasoline,where 87 octane gasoline contains 32 MJ per liter.How many food calories are there in one such 0.25-liter bottle of orange juice?
A)130 food calories
B)125 food calories
C)135 food calories
D)140 food calories
E)120 food calories
A)130 food calories
B)125 food calories
C)135 food calories
D)140 food calories
E)120 food calories

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
38
How much heat is required to turn a 1 kg cube of ice at -10 C to steam at 110 C?
A)3013 kJ
B)3055 kJ
C)2260 kJ
D)2510 kJ
E)2817 kJ
A)3013 kJ
B)3055 kJ
C)2260 kJ
D)2510 kJ
E)2817 kJ

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
39
A human body transports heat energy from the interior tissues,at temperature 37.0 C,to the skin surface at temperature 27.0 C.If the skin area is 1.5 m2 and its thickness is average 2.4 mm,what is the rate energy is transferred through the skin? Assume and effective thermal conductivity of skin of 0.022 W/(m * K)
A)70 W
B)100 W
C)120 W
D)170 W
E)140 W
A)70 W
B)100 W
C)120 W
D)170 W
E)140 W

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
40
A human body transports heat energy from the interior tissues,at temperature 37.0 C,to the skin surface at temperature 27.0 C,at a rate of 100 W.If the skin area is 1.5 m2 and its thickness is 3.0 mm,what is the effective thermal conductivity of skin?
A)7.1 * 10-4 W/(m * K)
B)0.020 W/(m * K)
C)0.74 W/(m * K)
D)1400 W/(m * K)
E)5000 W/(m * K)
A)7.1 * 10-4 W/(m * K)
B)0.020 W/(m * K)
C)0.74 W/(m * K)
D)1400 W/(m * K)
E)5000 W/(m * K)

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following statements is false?
A)Positive work done on a system always increases its internal energy.
B)Positive work done by a system always decreases its internal energy.
C)If you use your hands to squeeze a gas filled piston,the energy of the gas in the piston will increase.
D)In an adiabatic process,positive work done on a system is always equal to its gain in internal energy.
E)All of the choices are true.
A)Positive work done on a system always increases its internal energy.
B)Positive work done by a system always decreases its internal energy.
C)If you use your hands to squeeze a gas filled piston,the energy of the gas in the piston will increase.
D)In an adiabatic process,positive work done on a system is always equal to its gain in internal energy.
E)All of the choices are true.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
42
A gas in a cylinder with a piston that slides freely within is adiabatically expanded from 1.5 to 1.7 m3.If the pressure exerted by the walls on its container during the expansion was 2.0 atm,what heat must have been supplied to the gas in order to affect this expansion?
A)0
B)+41 kJ
C)-41 kJ
D)+65 kJ
E)-65 kJ
A)0
B)+41 kJ
C)-41 kJ
D)+65 kJ
E)-65 kJ

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
43
A gas in a cylinder with a piston that slides freely within is adiabatically expanded from 1.5 to 1.7 m3.If the pressure exerted by the walls on its container during the expansion was 2.0atm,by what amount did the internal energy of the gas change during this expansion?
A)+41 kJ
B)-41 kJ
C)+65 kJ
D)-65 kJ
E)0J
A)+41 kJ
B)-41 kJ
C)+65 kJ
D)-65 kJ
E)0J

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
44
How much heat is required to boil away 2.0 liter water that is at 100 C? ( Lv = 540 kcal/kg for water)
A)1080 kcal
B)270 kcal
C)2160 kcal
D)0 kcal
E)200 kcal
A)1080 kcal
B)270 kcal
C)2160 kcal
D)0 kcal
E)200 kcal

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
45
In a nuclear power plant the power of steam turns turbines to generate electricity.The process also generates a large amount of waste heat that needs to be removed from the system.Suppose a nuclear power plant generates waste heat at a rate of about 600 MW which is carried away by water pumped from a nearby lake.Given the specific heat of water 4190 J/kg K.and the increase of the temperature of the water from the lake by 12 C,the rate of required flow of water would be
A)1.2 * 104 kg/s.
B)3.5 * 104 kg/s.
C)7.5 * 105 kg/s
D)8.1 * 105 kg/s.
E)9.8 * 105 kg/s
A)1.2 * 104 kg/s.
B)3.5 * 104 kg/s.
C)7.5 * 105 kg/s
D)8.1 * 105 kg/s.
E)9.8 * 105 kg/s

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
46
A person tries to heat up her bath water by adding 5 liters of water at T = 82 C to 60 liters of water at T = 30 C.What is the final temperature of the water?
A)31 C
B)32 C
C)33 C
D)34 C
E)35 C
A)31 C
B)32 C
C)33 C
D)34 C
E)35 C

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
47
The power radiated by a black body (emissivity=1)of surface area 0.5 m2 is at a temperature of 40 C,while its surroundings are at a temperature of 20 C.What is the net power radiated by this object? (The Stefan-Boltzmann constant has a value of 5.67 * 10-8 W/m2 K4).
A)32.2 W
B)63.2 W
C)112.2 W
D)253.1 W
E)273.1 W
A)32.2 W
B)63.2 W
C)112.2 W
D)253.1 W
E)273.1 W

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
48
The tungsten filament inside a light bulb is heated to a temperature of 1250 K.It has a surface area 2 cm2.What is the net power radiated by the light bulb at room temperature (25 C)? (emissivity of tungsten is 0.7)
A)3 W
B)12 W
C)19 W
D)52 W
E)100 W
A)3 W
B)12 W
C)19 W
D)52 W
E)100 W

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
49
The amount of heat (in cal)needed to bring 1 g of ice from T = -20 to 100 C and completely evaporate it into steam is
A)120 cal.
B)427 cal
C)680 cal
D)730 cal
E)980 cal.
A)120 cal.
B)427 cal
C)680 cal
D)730 cal
E)980 cal.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
50
A 450-g steel ball with a temperature of 300 degrees Celsius is travelling at 290 m/s when it enters 90.0 liters of water at 20.0 degrees Celsius.Assuming the water stops the ball,what is the final temperature of the water after the ball and the water reach thermal equilibrium?
A)20.1 degrees Celsius
B)20.2 degrees Celsius
C)21.0 degrees Celsius
D)21.3 degrees Celsius
E)21.4 degrees Celsius
A)20.1 degrees Celsius
B)20.2 degrees Celsius
C)21.0 degrees Celsius
D)21.3 degrees Celsius
E)21.4 degrees Celsius

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
51
A 10-g ice cube at -10 C is dropped into 40 g of water at 30 C.After enough time has passed to allow the ice cube and water to come into thermal equilibrium,what is the temperature of the water?
A)-2.0 degrees Celsius
B)0.0 degrees Celsius
C)7.0 degrees Celsius
D)10.0 degrees Celsius
E)22.0 degrees Celsius
A)-2.0 degrees Celsius
B)0.0 degrees Celsius
C)7.0 degrees Celsius
D)10.0 degrees Celsius
E)22.0 degrees Celsius

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
52
A gas is in a cylinder undergoes a quasi-static process such that P = V2,starting with a volume of 2.0 m3.If the constant has a value of 7.4Nm-8and the work done on the gas is 17.2 J,what is the final volume of the gas?
A)0.32 m3
B)1.0 m3
C)1.5 m3
D)2.3 m3
A)0.32 m3
B)1.0 m3
C)1.5 m3
D)2.3 m3

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
53
A container holding 0.25 kg of water at 20.0 C is placed into the freezer.How much energy must be removed from the water to turn it into ice?
A)104 kJ
B)83.5 kJ
C)20.9 kJ
D)15.2 kJ
E)3.58 kJ
A)104 kJ
B)83.5 kJ
C)20.9 kJ
D)15.2 kJ
E)3.58 kJ

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
54
The total energy required to get 1 kg of water from a temperature of 90 C to 120 C is (latent heat of vaporization is 540 kcal/kg for water;and specific heats are 1.0 kcal/(kg C)for water,and 0.48 kcal/(kg C)for steam).
A)505.9 kcal
B)510.0 kcal
C)520.4 kcal
D)540.0 kcal
E)559.6 kcal
A)505.9 kcal
B)510.0 kcal
C)520.4 kcal
D)540.0 kcal
E)559.6 kcal

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
55
If a donut contains 250 food calories,express the kinetic energy of a 1200-kg car speeding along the highway at 65 mph in units of donuts.
A)480
B)2.0
C)0.48
D)2.4
A)480
B)2.0
C)0.48
D)2.4

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
56
Mars is 1.52 times farther away from the Sun than the Earth and has a diameter that is 0.532 times that of the Earth.If the solar irradiance of the Sun on the Earth is 1400 W/m2,what is the solar irradiance on the surface of Mars?
A)170 W/m2
B)490 W/m2
C)610 W/m2
D)920 W/m2
E)1100 W/m2
A)170 W/m2
B)490 W/m2
C)610 W/m2
D)920 W/m2
E)1100 W/m2

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
57
A gas is in a cylinder is compressed in a quasi-static process such that P = (\beta\)V2 from a volume of 2.0 m3 down to 1.0m3.If 17.2 J of work is required,what value does the constant have?
A)5.8 Nm-8
B)7.4 Nm-8
C)11 Nm-8
D)17 Nm-8
A)5.8 Nm-8
B)7.4 Nm-8
C)11 Nm-8
D)17 Nm-8

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
58
A block of aluminum with a mass of 2.6 kg is at 13 C and comes in contact with a hot block of copper with a mass of 10.2 kg at 93 C.What is the final temperature? (caluminum = 0.22 kcal/kg C;ccopper = 0.093 kcal/kg C)
A)19 C
B)21 C
C)50 C
D)63 C
E)83 C
A)19 C
B)21 C
C)50 C
D)63 C
E)83 C

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
59
An 8.2-kg block of copper at 80 C is dropped into a container with 0.62 L of water at 10 C.How much energy in the form of heat is absorbed by the water as the copper and water reach thermal equilibrium?
A)100 kJ
B)130 kJ
C)180 kJ
D)220 kJ
E)250 kJ
A)100 kJ
B)130 kJ
C)180 kJ
D)220 kJ
E)250 kJ

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
60
One of the glass windows (k = 0.80 W/m C)in your house has an area of 1.21 m2 and a thickness of 5 mm.When the temperature difference between its surfaces is 25 C,the rate of energy transferred through the window is
A)2.66 kW.
B)3.13 kW.
C)4.84 kW.
D)6.98 kW.
E)7.32 kW.
A)2.66 kW.
B)3.13 kW.
C)4.84 kW.
D)6.98 kW.
E)7.32 kW.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
61
The temperature of the Sun at its surface is 5800 K and has a power output of 4.0·1026 W.Estimate the Sun's current surface area.Assume the Sun is a perfect blackbody with emissivity = 1.(The Stefan-Boltzmann constant is = 5.67 * 10-8 W/m2K2.)
A)2.5 * 1018 m2
B)4.2 * 1018 m2
C)4.5 * 1017 m2
D)6.2 * 1018 m2
E)7.5 * 1017 m2
A)2.5 * 1018 m2
B)4.2 * 1018 m2
C)4.5 * 1017 m2
D)6.2 * 1018 m2
E)7.5 * 1017 m2

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
62
A scientist is performing a calorimetric test with an 800-g sample of an unknown substance.She finds that the sample absorbs 152 calories for every ºC it rises in temperature while in its solid phase.What might the substance be?
A)granite: c = 0.19 kcal/kg·ºC
B)copper: c = 0.09 kcal/kg·ºC
C)lead: c = 0.03 kcal/kg·ºC
D)iron: c = 0.11 kcal/kg·ºC
E)wood: c = 0.40 kcal/kg·ºC
A)granite: c = 0.19 kcal/kg·ºC
B)copper: c = 0.09 kcal/kg·ºC
C)lead: c = 0.03 kcal/kg·ºC
D)iron: c = 0.11 kcal/kg·ºC
E)wood: c = 0.40 kcal/kg·ºC

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
63
The temperature of the Sun at its surface is 5800 K and has a power output of 4.0·1026 W.Estimate the Sun's current radius.Assume the Sun is a perfect blackbody with emissivity = 1.(The Stefan-Boltzmann constant is = 5.67 * 10-8 W/m2K2.)
A)6.6 * 108 m
B)6.2 * 108 m
C)8.9 * 108 m
D)2.5 * 109 m
E)7.0 * 108 m
A)6.6 * 108 m
B)6.2 * 108 m
C)8.9 * 108 m
D)2.5 * 109 m
E)7.0 * 108 m

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
64
An immersion heater with a power rating of 600 W is used to heat water.If the heater is placed in 1.9 L of water at room temperature (20 C),what is the temperature of the water after 12 minutes?
A)87 C
B)67 C
C)77 C
D)74 C
E)57 C
A)87 C
B)67 C
C)77 C
D)74 C
E)57 C

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
65
An immersion heater with a power rating of 600 W is used to heat water.How long should it take to bring 1.9 L of water from room temperature (20 C)to 87 C?
A)4.8 min
B)10.8 min
C)14.8 min
D)16.6 min
E)18.6 min
A)4.8 min
B)10.8 min
C)14.8 min
D)16.6 min
E)18.6 min

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
66
The temperature of the Sun at its surface is 5800 K and has a radius of 7.0 * 108 m.Estimate the Sun's current power output.Assume the Sun is a perfect blackbody with emissivity = 1.(The Stefan-Boltzmann constant is = 5.67 *10-8 W/m2K2.)
A)4.0·1026 W
B)4.0·1026 W.
C)3.2·1026 W
D)2.0·1023 W
E)6.2·1026 W
A)4.0·1026 W
B)4.0·1026 W.
C)3.2·1026 W
D)2.0·1023 W
E)6.2·1026 W

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
67
In the process shown,about how much work in J is done by the system in going from point A to point B? 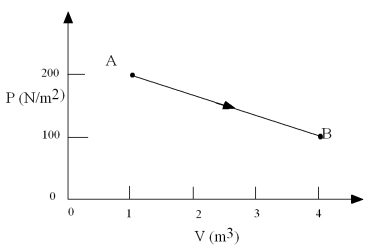
A)450
B)800
C)-800
D)-450
E)0

A)450
B)800
C)-800
D)-450
E)0

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck



