Deck 29: Atoms and Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/105
Play
Full screen (f)
Deck 29: Atoms and Molecules
1
Which of the following values can be taken by the electron spin quantum number, ms?
A) ±1/2
B) 0
C) ±1
D) ±2
E) ±3
A) ±1/2
B) 0
C) ±1
D) ±2
E) ±3
A
2
The principal quantum number n can have any integer value ranging from
A) -∞ to +∞.
B) 0 to ∞.
C) 1 to ∞.
D) 1 to 100.
A) -∞ to +∞.
B) 0 to ∞.
C) 1 to ∞.
D) 1 to 100.
C
3
Which of the following statements are true for the Bohr model of the atom? (There could be more than one correct choice.)
A) The spacing between all the electron shells is the same.
B) The energy difference between all the electron shells is the same.
C) As we look at higher and higher electron shells, they get closer and closer together, but the difference in energy between them gets greater and greater.
D) As we look at higher and higher electron shells, they get farther and farther apart, but the difference in energy between them gets smaller and smaller.
E) There is no general pattern in the spacing of the shells or their energy differences.
A) The spacing between all the electron shells is the same.
B) The energy difference between all the electron shells is the same.
C) As we look at higher and higher electron shells, they get closer and closer together, but the difference in energy between them gets greater and greater.
D) As we look at higher and higher electron shells, they get farther and farther apart, but the difference in energy between them gets smaller and smaller.
E) There is no general pattern in the spacing of the shells or their energy differences.
D
4
The Lyman series is formed by electron transitions in hydrogen that
A) end on the n = 1 shell.
B) begin on the n = 1 shell.
C) end on the n = 2 shell.
D) begin on the n = 2 shell.
E) are between the n = 1 and n = 3 shells.
A) end on the n = 1 shell.
B) begin on the n = 1 shell.
C) end on the n = 2 shell.
D) begin on the n = 2 shell.
E) are between the n = 1 and n = 3 shells.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
5
The orbital angular momentum quantum number can take which of the following values for any given value of the principal quantum number, n?
A) ℓ = 0, 1, 2, . . .
B) ℓ = 0, 1, 2, . . . , n
C) ℓ = 0, 1, 2, . . . , (n - 1)
D) ℓ = 1, 2, 3, 4, . . .
E) ℓ = 1, 2, 3, 4, . . ., (n + 1)
A) ℓ = 0, 1, 2, . . .
B) ℓ = 0, 1, 2, . . . , n
C) ℓ = 0, 1, 2, . . . , (n - 1)
D) ℓ = 1, 2, 3, 4, . . .
E) ℓ = 1, 2, 3, 4, . . ., (n + 1)

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
6
According to the quantum mechanical model of the hydrogen atom, if the orbital angular momentum quantum number is ℓ, there will be how many permitted magnetic quantum numbers?
A) ℓ/2
B) 2ℓ
C) 2ℓ + 1
D) 2ℓ - 1
E) 3ℓ
A) ℓ/2
B) 2ℓ
C) 2ℓ + 1
D) 2ℓ - 1
E) 3ℓ

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
7
The energy difference between adjacent orbit radii in a hydrogen atom
A) increases with increasing values of n.
B) decreases with increasing values of n.
C) remains constant for all values of n.
D) varies randomly with increasing values of n.
A) increases with increasing values of n.
B) decreases with increasing values of n.
C) remains constant for all values of n.
D) varies randomly with increasing values of n.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
8
The distance between adjacent orbits in a hydrogen atom
A) increases with increasing values of n.
B) decreases with increasing values of n.
C) remains constant for all values of n.
D) varies randomly with increasing values of n.
A) increases with increasing values of n.
B) decreases with increasing values of n.
C) remains constant for all values of n.
D) varies randomly with increasing values of n.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
9
To which of the following values of n does the shortest wavelength in the Balmer series correspond?
A) 3
B) 5
C) 7
D) 1
E) ∞ (very large)
A) 3
B) 5
C) 7
D) 1
E) ∞ (very large)

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
10
Hydrogen atoms can emit four spectral lines with visible colors from red to violet. These four visible lines emitted by hydrogen atoms are produced by electrons
A) that start in the n = 2 level.
B) that end up in the n = 2 level.
C) that end up in the n = 3 level.
D) that end up in the ground state.
E) that start in the ground state.
A) that start in the n = 2 level.
B) that end up in the n = 2 level.
C) that end up in the n = 3 level.
D) that end up in the ground state.
E) that start in the ground state.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
11
When an electron jumps from an orbit where n = 4 to one where n = 2
A) a photon is emitted.
B) a photon is absorbed.
C) two photons are emitted.
D) two photons are absorbed.
E) None of the given answers are correct.
A) a photon is emitted.
B) a photon is absorbed.
C) two photons are emitted.
D) two photons are absorbed.
E) None of the given answers are correct.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
12
If a hydrogen atom originally in a state with principal quantum number n is excited to state n' = 2n, then
A) its radius and binding energy will double.
B) its radius will quadruple and the binding energy will double.
C) its radius will double and the binding energy will quadruple.
D) its radius will quadruple and the binding energy will be reduced by a factor of four.
E) its radius and binding energy will quadruple.
A) its radius and binding energy will double.
B) its radius will quadruple and the binding energy will double.
C) its radius will double and the binding energy will quadruple.
D) its radius will quadruple and the binding energy will be reduced by a factor of four.
E) its radius and binding energy will quadruple.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
13
To which of the following values of n does the longest wavelength in the Balmer series correspond?
A) 3
B) 5
C) 1
D) 7
E) ∞ (very large)
A) 3
B) 5
C) 1
D) 7
E) ∞ (very large)

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
14
According to Pauli's exclusion principle, how many electrons in an atom may have a particular set of quantum numbers?
A) 1
B) 3
C) 2
D) 4
E) 5
A) 1
B) 3
C) 2
D) 4
E) 5

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
15
According to the quantum mechanical model of the hydrogen atom, if the principal quantum number is n, how many different orbital angular momentum quantum numbers are permitted?
A) n/2
B) n
C) 2n
D) 3n
E) 4n
A) n/2
B) n
C) 2n
D) 3n
E) 4n

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
16
The orbital angular momentum quantum number ℓ can have any integer value ranging from
A) 0 to n.
B) 0 to (n-1).
C) 1 to n.
D) 1 to (n+1).
E) -n to n.
A) 0 to n.
B) 0 to (n-1).
C) 1 to n.
D) 1 to (n+1).
E) -n to n.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
17
The figure shows part of the energy level diagram of a certain atom. The energy spacing between levels 1 and 2 is twice that between 2 and 3. If an electron makes a transition from level 3 to level 2, the radiation of wavelength λ is emitted. What possible radiation wavelengths might be produced by other transitions between the three energy levels? 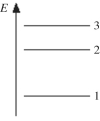
A) both λ/2 and λ/3
B) only λ/2
C) both 2λ and 3λ
D) only 2λ

A) both λ/2 and λ/3
B) only λ/2
C) both 2λ and 3λ
D) only 2λ

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
18
The Paschen series is formed by electron transitions that
A) end on the n = 1 shell.
B) begin on the n = 1 shell.
C) end on the n = 2 shell.
D) begin on the n = 3 shell.
E) end on the n = 3 shell.
A) end on the n = 1 shell.
B) begin on the n = 1 shell.
C) end on the n = 2 shell.
D) begin on the n = 3 shell.
E) end on the n = 3 shell.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
19
The Balmer series is formed by electron transitions in hydrogen that
A) end on the n = 1 shell.
B) begin on the n = 1 shell.
C) end on the n = 2 shell.
D) begin on the n = 2 shell.
E) are between the n = 1 and n = 3 shells.
A) end on the n = 1 shell.
B) begin on the n = 1 shell.
C) end on the n = 2 shell.
D) begin on the n = 2 shell.
E) are between the n = 1 and n = 3 shells.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
20
A hydrogen atom is in the 6h state. What is the principal quantum number.
A) 0
B) 3
C) 5
D) 6
E) 7
A) 0
B) 3
C) 5
D) 6
E) 7

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
21
The magnetic quantum number m1 can have any integer value ranging from
A) -n to +n.
B) -ℓ to +ℓ.
C) 0 to n.
D) 0 to ℓ.
E) 0 to (n-1)
A) -n to +n.
B) -ℓ to +ℓ.
C) 0 to n.
D) 0 to ℓ.
E) 0 to (n-1)

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
22
What is the wavelength in the Balmer series for n = 15?
A) 277.1 nm
B) 371.1 nm
C) 188.6 nm
D) 656 nm
E) 754.2 nm
A) 277.1 nm
B) 371.1 nm
C) 188.6 nm
D) 656 nm
E) 754.2 nm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
23
If ℓ = 4, which one of the following is a possible quantum number for n?
A) 0
B) 2
C) 3
D) 4
E) 8
A) 0
B) 2
C) 3
D) 4
E) 8

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
24
What is the value of n in the Balmer series for which the wavelength is 410.2 nm.
A) 4
B) 5
C) 6
D) 7
E) 9
A) 4
B) 5
C) 6
D) 7
E) 9

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
25
If n = 5, which one of the following is not an allowed magnetic quantum number m1?
A) 0
B) 2
C) 4
D) 5
A) 0
B) 2
C) 4
D) 5

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
26
The elements in the periodic table that have completely filled shells or subshells are referred to as
A) noble gases.
B) halogens.
C) alkali metals.
D) transition elements.
A) noble gases.
B) halogens.
C) alkali metals.
D) transition elements.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
27
What value of n corresponds to a wavelength of 91.7 nm in the Lyman series?
A) 1
B) 3
C) 5
D) 9
E) 13
A) 1
B) 3
C) 5
D) 9
E) 13

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
28
One of the emission lines described by the original version of Balmer's formula has wavelength 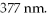 What is the value of n in Balmer's formula that gives this emission line?
What is the value of n in Balmer's formula that gives this emission line?
A) 11
B) 12
C) 13
D) 14
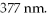 What is the value of n in Balmer's formula that gives this emission line?
What is the value of n in Balmer's formula that gives this emission line?A) 11
B) 12
C) 13
D) 14

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
29
In its ground state, the quantum numbers (n, ℓ, m1, ms) for hydrogen are, respectively,
A) 1, 1, 1, 1.
B) 1, 0, 0, 0.
C) 1, 0, 0, ±1/2.
D) 1, 1, 1, ±1/2.
A) 1, 1, 1, 1.
B) 1, 0, 0, 0.
C) 1, 0, 0, ±1/2.
D) 1, 1, 1, ±1/2.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
30
What is the longest wavelength in the Paschen series?
A) 2.01 nm
B) 2.01 μm
C) 365 nm
D) 1.88 nm
E) 1.88 μm
A) 2.01 nm
B) 2.01 μm
C) 365 nm
D) 1.88 nm
E) 1.88 μm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
31
Consider ground-state helium having two electrons in orbit. If one of the electrons has quantum numbers (n, ℓl, m1, ms) of 1, 0, 0, -1/2 respectively, the quantum numbers for the other electron will be
A) 1, 1, 0, -1/2.
B) 1, 0, 0, +1/2.
C) 1, 1, 1, +1/2.
D) none of the given answers.
A) 1, 1, 0, -1/2.
B) 1, 0, 0, +1/2.
C) 1, 1, 1, +1/2.
D) none of the given answers.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
32
What is the longest wavelength in the Lyman Series?
A) 45.60 nm
B) 91.20 nm
C) 121.5 nm
D) 240.1 nm
E) 365 nm
A) 45.60 nm
B) 91.20 nm
C) 121.5 nm
D) 240.1 nm
E) 365 nm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
33
The value of a wavelength in the Balmer series is 372.1 nm. What is the corresponding value of n?
A) 6
B) 3
C) 9
D) 10
E) 14
A) 6
B) 3
C) 9
D) 10
E) 14

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
34
What value of n corresponds to a wavelength of 922.7 nm in the Paschen series?
A) 3
B) 5
C) 7
D) 9
E) 15
A) 3
B) 5
C) 7
D) 9
E) 15

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
35
What is the longest wavelength in the Balmer series?
A) 240 nm
B) 328 nm
C) 365 nm
D) 656 nm
E) 820 nm
A) 240 nm
B) 328 nm
C) 365 nm
D) 656 nm
E) 820 nm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
36
What is the shortest wavelength of the Lyman series?
A) 91.16 nm
B) 45.60 nm
C) 121.5 nm
D) 204.1 nm
E) 365 nm
A) 91.16 nm
B) 45.60 nm
C) 121.5 nm
D) 204.1 nm
E) 365 nm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
37
What is the shortest wavelength in the Paschen series?
A) 410.2 nm
B) 410.2 μm
C) 365 nm
D) 820.4 nm
E) 820.4 μm
A) 410.2 nm
B) 410.2 μm
C) 365 nm
D) 820.4 nm
E) 820.4 μm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
38
The electron spin quantum number can have values of
A) -1/2, -1, 0, +1, +1/2.
B) -1/2, -1, +1, +1/2.
C) -1/2, 0, +1/2.
D) -1/2, +1/2.
E) only +1/2.
A) -1/2, -1, 0, +1, +1/2.
B) -1/2, -1, +1, +1/2.
C) -1/2, 0, +1/2.
D) -1/2, +1/2.
E) only +1/2.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
39
What is the shortest wavelength in the Balmer series?
A) 328 nm
B) 365 nm
C) 456 nm
D) 656 nm
E) 820 nm
A) 328 nm
B) 365 nm
C) 456 nm
D) 656 nm
E) 820 nm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
40
What is the atomic number of a neutral atom that has an electron configuration of 1s2 2s2 2p6 3s2 3p2?
A) 5
B) 11
C) 14
D) 20
A) 5
B) 11
C) 14
D) 20

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
41
A hydrogen atom makes a downward transition from the 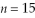 state to the
state to the 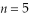 state. Find the wavelength of the emitted photon. (c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J, h = 6.626 × 10-34 J ∙ s)
state. Find the wavelength of the emitted photon. (c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J, h = 6.626 × 10-34 J ∙ s)
A) 2.56 μm
B) 1.54 μm
C) 2.05 μm
D) 3.07 μm
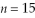 state to the
state to the 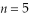 state. Find the wavelength of the emitted photon. (c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J, h = 6.626 × 10-34 J ∙ s)
state. Find the wavelength of the emitted photon. (c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J, h = 6.626 × 10-34 J ∙ s)A) 2.56 μm
B) 1.54 μm
C) 2.05 μm
D) 3.07 μm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
42
What is the energy of the photon emitted when an electron drops from the 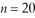 state to the
state to the 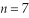 state in a hydrogen atom?
state in a hydrogen atom?
A) 0.244 eV
B) 0.264 eV
C) 0.283 eV
D) 0.303 eV
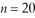 state to the
state to the 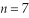 state in a hydrogen atom?
state in a hydrogen atom?A) 0.244 eV
B) 0.264 eV
C) 0.283 eV
D) 0.303 eV

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
43
The shortest wavelength of a photon that can be emitted by a hydrogen atom, for which the initial state is 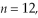 is closest to which one of the following values?
is closest to which one of the following values? 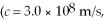
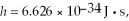
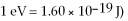
A) 92 nm
B) 82 nm
C) 72 nm
D) 62 nm
E) 52 nm
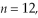 is closest to which one of the following values?
is closest to which one of the following values? 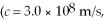
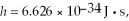
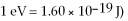
A) 92 nm
B) 82 nm
C) 72 nm
D) 62 nm
E) 52 nm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
44
In a hydrogen atom, the electron makes a transition from the n = 8 to the n = 3 state. The wavelength of the emitted photon is closest to which one of the following values? (c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J, h = 6.626 × 10-34 J ∙ s)
A) 9.57 × 10-7 m
B) 1.13 × 10-6 m
C) 3.12 × 10-7 m
D) 4.52 × 10-6 m
E) 6.34 × 10-7 m
A) 9.57 × 10-7 m
B) 1.13 × 10-6 m
C) 3.12 × 10-7 m
D) 4.52 × 10-6 m
E) 6.34 × 10-7 m

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
45
Given that the binding energy of the hydrogen atom in its ground state is -13.6 eV, what is the energy when it is in the n = 5 state?
A) 2.72 eV
B) -2.72 eV
C) 0.544 eV
D) -0.544 eV
A) 2.72 eV
B) -2.72 eV
C) 0.544 eV
D) -0.544 eV

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
46
The longest wavelength of a photon that can be emitted by a hydrogen atom, for which the initial state is n = 3, is closest to which one of the following values? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 550 nm
B) 575 nm
C) 600 nm
D) 625 nm
E) 658 nm
A) 550 nm
B) 575 nm
C) 600 nm
D) 625 nm
E) 658 nm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
47
Given that the energy levels of the hydrogen atom are given by En = 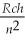 , where R = 1.097 × 107 m-1, what wavelength photon is emitted when the atom undergoes a transition from the n = 4 to the n = 6 level?
, where R = 1.097 × 107 m-1, what wavelength photon is emitted when the atom undergoes a transition from the n = 4 to the n = 6 level?
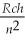 , where R = 1.097 × 107 m-1, what wavelength photon is emitted when the atom undergoes a transition from the n = 4 to the n = 6 level?
, where R = 1.097 × 107 m-1, what wavelength photon is emitted when the atom undergoes a transition from the n = 4 to the n = 6 level?
Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
48
A hydrogen atom is excited to the n = 9 level. Its decay to the n = 6 level detected in a photographic plate. What is the frequency of the light photographed? (1 eV = 1.60 × 10-19 J, h = 6.626 × 10-34 J ∙ s)
A) 5.08 × 1013 Hz
B) 5910 Hz
C) 5910 nm
D) 3.28 × 10-9 km
A) 5.08 × 1013 Hz
B) 5910 Hz
C) 5910 nm
D) 3.28 × 10-9 km

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
49
A hydrogen atom is excited to the n = 11 level. Its decay to the n = 7 level is detected in a photographic plate. What is the wavelength of the light photographed? (c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J, h = 6.626 × 10-34 J ∙ s)
A) 7510 nm
B) 4670 nm
C) 12,400 nm
D) 4380 nm
A) 7510 nm
B) 4670 nm
C) 12,400 nm
D) 4380 nm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
50
The longest wavelength photon that can be emitted by a hydrogen atom, for which the final state is 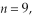 is closest to which one of the following values? (c = 3.0 × 108 m/s,
is closest to which one of the following values? (c = 3.0 × 108 m/s, 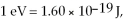
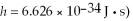
A) 39,000 nm
B) 22,000 nm
C) 7400 nm
D) 16,000 nm
E) 28,000 nm
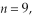 is closest to which one of the following values? (c = 3.0 × 108 m/s,
is closest to which one of the following values? (c = 3.0 × 108 m/s, 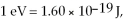
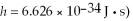
A) 39,000 nm
B) 22,000 nm
C) 7400 nm
D) 16,000 nm
E) 28,000 nm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
51
What is the energy required to remove the electron from a hydrogen atom in the n = 11 state?
A) 0.112 eV
B) 1.24 eV
C) 13.5 eV
D) 12.4 eV
E) 0.141 eV
A) 0.112 eV
B) 1.24 eV
C) 13.5 eV
D) 12.4 eV
E) 0.141 eV

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
52
Light shines through atomic hydrogen gas that was initially in its ground state. You observe that after awhile much of the hydrogen gas has been excited to its n = 5 state. What wavelength of light entering the gas caused this excitation? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 110 nm
B) 91.4 nm
C) 95.2 nm
D) 2280 nm
A) 110 nm
B) 91.4 nm
C) 95.2 nm
D) 2280 nm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
53
If a hydrogen atom in the ground state absorbs a photon of energy 12.09 eV, to which state will the electron make a transition?
A) n = 2
B) n = 3
C) n = 4
D) n = 5
A) n = 2
B) n = 3
C) n = 4
D) n = 5

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
54
What is the ionization energy of the neutral hydrogen atom?
A) 27.2 eV
B) 13.6 eV
C) 6.8 eV
D) none of the given answers
A) 27.2 eV
B) 13.6 eV
C) 6.8 eV
D) none of the given answers

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
55
The wavelength of a ruby laser is 694.3 nm. What is the energy difference between the two energy states for the transition that produces this light? (c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J, h = 6.626 × 10-34 J ∙ s)
A) 1.54 eV
B) 1.65 eV
C) 1.79 eV
D) 1.81 eV
A) 1.54 eV
B) 1.65 eV
C) 1.79 eV
D) 1.81 eV

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
56
What is the energy (in electron-volts) of the n = 3 state of atomic hydrogen?

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
57
If light excites atomic hydrogen from its lowest energy level to the n = 12 level, what is the energy of the photons of this light?
A) 13.5 eV
B) 32.2 eV
C) 13.6 eV
D) 0.0944 eV
E) 1.13 eV
A) 13.5 eV
B) 32.2 eV
C) 13.6 eV
D) 0.0944 eV
E) 1.13 eV

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
58
What is the shortest wavelength of a photon that can be emitted by a hydrogen atom, for which the initial state is n = 3? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 822 nm
B) 850 nm
C) 103 nm
D) 91.4 nm
E) 950 nm
A) 822 nm
B) 850 nm
C) 103 nm
D) 91.4 nm
E) 950 nm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
59
A hydrogen atom is in its n = 2 excited state when its electron absorbs 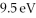 in an interaction with a photon. What is the energy of the resulting free electron?
in an interaction with a photon. What is the energy of the resulting free electron?
A) 6.1 eV
B) 7.9 eV
C) 8.2 eV
D) 9.2 eV
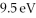 in an interaction with a photon. What is the energy of the resulting free electron?
in an interaction with a photon. What is the energy of the resulting free electron?A) 6.1 eV
B) 7.9 eV
C) 8.2 eV
D) 9.2 eV

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
60
Consider the Bohr model for the hydrogen atom. (c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J, h = 6.626 × 10-34 J ∙ s)
(a) How much energy (in eV) is needed to cause a transition of an electron from the second excited state to the third excited state?
(b) What wavelength photon just has enough energy to initiate the transition in (a)?
(a) How much energy (in eV) is needed to cause a transition of an electron from the second excited state to the third excited state?
(b) What wavelength photon just has enough energy to initiate the transition in (a)?

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
61
A hydrogen atom with a barely bound electron may have an average radius as large as a bacterium, which is a radius of 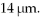 What is the nearest principal quantum number of the atom in this state? The radius for ground state hydrogen is 0.0529 nm.
What is the nearest principal quantum number of the atom in this state? The radius for ground state hydrogen is 0.0529 nm.
A) 514
B) 51
C) 16
D) 264,650
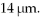 What is the nearest principal quantum number of the atom in this state? The radius for ground state hydrogen is 0.0529 nm.
What is the nearest principal quantum number of the atom in this state? The radius for ground state hydrogen is 0.0529 nm.A) 514
B) 51
C) 16
D) 264,650

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
62
What is the wavelength of the photon emitted when an electron in a hydrogen atom which is in the initial state n = 8 jumps to the final state n = 2? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 205 nm
B) 104 nm
C) 389 nm
D) 486 nm
E) 610 nm
A) 205 nm
B) 104 nm
C) 389 nm
D) 486 nm
E) 610 nm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
63
The only valid electron state and shell designation among the following is
A) 1p, K (n = 1) shell.
B) 2s, K (n = 1) shell.
C) 1s, L (n = 2) shell.
D) 2p, L (n = 2) shell.
E) 3f, M (n = 3) shell.
A) 1p, K (n = 1) shell.
B) 2s, K (n = 1) shell.
C) 1s, L (n = 2) shell.
D) 2p, L (n = 2) shell.
E) 3f, M (n = 3) shell.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
64
An atom has completely filled inner shells and a single valence electron in an excited p state. The filled inner shells have an orbital momentum equal to zero. What is the magnitude of the orbital angular momentum of the atom?
A) 1.0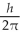
B) 1.2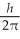
C) 1.4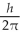
D) 1.7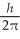
E) 2.0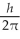
A) 1.0
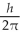
B) 1.2
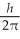
C) 1.4
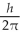
D) 1.7
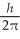
E) 2.0
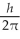

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
65
Write out the electron configuration for the ground state of the phosphorus atom, which has 15 electrons.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
66
For an electron in the M (n = 3) shell, find (a) the largest possible orbital angular momentum it can have, and (b) the smallest possible orbital angular momentum it can have. Express your answers in SI units, and for each case indicate the subshell (s, p, d, ...) of the electron. (h = 6.626 × 10-34 J ∙ s)

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
67
Consider the n = 10 shell.
(a) What is the largest value of the angular momentum quantum number, ℓ, in this shell?
(b) How many electrons can be placed in this shell?
(a) What is the largest value of the angular momentum quantum number, ℓ, in this shell?
(b) How many electrons can be placed in this shell?

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
68
An atom with atomic number 6 is in its ground state. How many electrons are in its outermost shell?

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
69
In a transition from one vibrational state to another, a molecule emits a photon of wavelength 5.56 µm. What is the energy difference between these two states? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 0.223 eV
B) 2.23 MeV
C) 13.6 eV
D) 13.6 MeV
E) 0.223 MeV
A) 0.223 eV
B) 2.23 MeV
C) 13.6 eV
D) 13.6 MeV
E) 0.223 MeV

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
70
In making a transition from state n = 1 to state n = 2, the hydrogen atom must
A) absorb a photon of energy 10.2 eV.
B) emit a photon of energy 10.2 eV.
C) absorb a photon of energy 13.58 eV.
D) emit a photon of energy 13.58 eV.
A) absorb a photon of energy 10.2 eV.
B) emit a photon of energy 10.2 eV.
C) absorb a photon of energy 13.58 eV.
D) emit a photon of energy 13.58 eV.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
71
What is the wavelength of the emitted photon if an electron in the hydrogen atom makes a transition from the n = 7 state to the n = 2 state? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 199 nm
B) 365 nm
C) 4480 nm
D) 398 nm
E) 796 nm
A) 199 nm
B) 365 nm
C) 4480 nm
D) 398 nm
E) 796 nm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
72
What is the greatest magnitude of the orbital angular momentum L that you can find in a state with 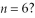
A) 5.48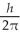
B) 5.92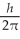
C) 6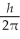
D) 6.48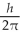
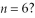
A) 5.48
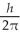
B) 5.92
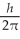
C) 6
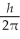
D) 6.48

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
73
In the n = 1 state, the energy of the hydrogen atom is -13.6 eV. What is its energy in the n = 2 state?
A) -6.79 eV
B) -4.53 eV
C) -3.40 eV
D) -1.51 eV
A) -6.79 eV
B) -4.53 eV
C) -3.40 eV
D) -1.51 eV

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
74
What is the wavelength of the photon emitted when an electron in a hydrogen atom which is in the initial state n = 4 jumps to the final state n = 2? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 243 nm
B) 486 nm
C) 556 nm
D) 312 nm
E) 609 nm
A) 243 nm
B) 486 nm
C) 556 nm
D) 312 nm
E) 609 nm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
75
What frequency must a photon have to raise an electron in a hydrogen atom from the n = 2 to the n = 4 state? (h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 3.06 × 1014 Hz
B) 6.16 × 1014 Hz
C) 4.11 × 1014 Hz
D) 5.20 × 1014 Hz
E) 9.24 × 1014 Hz
A) 3.06 × 1014 Hz
B) 6.16 × 1014 Hz
C) 4.11 × 1014 Hz
D) 5.20 × 1014 Hz
E) 9.24 × 1014 Hz

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
76
The wavelength of the emitted photon if an electron in the hydrogen atom makes a transition from the n = 2 state to the ground state is closest to which of the following values? (c = 3.0 × 108 m/s, h = 6.626 × 10-34 J ∙ s, 1 eV = 1.60 × 10-19 J)
A) 122 nm
B) 203 nm
C) 243 nm
D) 389 nm
E) 411 nm
A) 122 nm
B) 203 nm
C) 243 nm
D) 389 nm
E) 411 nm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
77
Consider the Bohr model for the hydrogen atom in its second excited state.
(a) Determine the binding energy (eV) of the electron.
(b) What is the radius of the electron orbit, given that r1 = 0.0529 nm?
(c) How far is it from the next higher excited orbit?
(a) Determine the binding energy (eV) of the electron.
(b) What is the radius of the electron orbit, given that r1 = 0.0529 nm?
(c) How far is it from the next higher excited orbit?

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
78
Given that the speed of an electron in the ground state of hydrogen is 2190 km/s, what is the speed of an electron in the n = 4 orbit of hydrogen?
A) 547 km/s
B) 31,000 km/s
C) 139 km/s
D) 178 km/s
E) 2180 km/s
A) 547 km/s
B) 31,000 km/s
C) 139 km/s
D) 178 km/s
E) 2180 km/s

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
79
Calculate the orbital Bohr radius of the 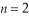 excited state in a hydrogen atom.
excited state in a hydrogen atom. 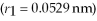
A) 0.212 nm
B) 0.106 nm
C) 0.170 nm
D) 0.244 nm
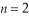 excited state in a hydrogen atom.
excited state in a hydrogen atom. 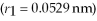
A) 0.212 nm
B) 0.106 nm
C) 0.170 nm
D) 0.244 nm

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
80
Radio astronomers often study the radiation emitted by a hydrogen atom from a transition between the two hyperfine levels associated with the ground state. This radiation has a wavelength of 21 cm. What is the energy difference between the hyperfine levels? (1 eV = 1.60 × 10-19 J)
A) 5.9 × 10-6 eV
B) 5.9 × 10-25 J
C) 1.7 × 10-24 J
D) 4.7 × 10-25 J
A) 5.9 × 10-6 eV
B) 5.9 × 10-25 J
C) 1.7 × 10-24 J
D) 4.7 × 10-25 J

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck



