Deck 3: Molecules,compounds,and Nomenclature
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/172
Play
Full screen (f)
Deck 3: Molecules,compounds,and Nomenclature
1
What is the empirical formula for Hg2(NO3)2?
A)Hg2(NO3)2
B)HgNO3
C)Hg(NO3)2
D)Hg2NO3
E)Hg4(NO3)4
A)Hg2(NO3)2
B)HgNO3
C)Hg(NO3)2
D)Hg2NO3
E)Hg4(NO3)4
HgNO3
2
Identify a possible molecular formula for a compound with an empirical formula C3H5ClO.
A)C6H10ClO2
B)C5H10Cl2O2
C)C6H10Cl2O2
D)C6H10O2
E)C6H12Cl2O2
A)C6H10ClO2
B)C5H10Cl2O2
C)C6H10Cl2O2
D)C6H10O2
E)C6H12Cl2O2
C6H10Cl2O2
3
Which of the following is a molecular element?
A)Mg
B)Ar
C)Xe
D)I
E)Li
A)Mg
B)Ar
C)Xe
D)I
E)Li
I
4
Which of the following contains both ionic and covalent bonds?
A)CaI2
B)CO2
C)CaSO4
D)SF6
E)C2H4
A)CaI2
B)CO2
C)CaSO4
D)SF6
E)C2H4

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is an atomic element?
A)Br
B)H
C)N
D)O
E)Mg
A)Br
B)H
C)N
D)O
E)Mg

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
6
A covalent bond is best described as
A)the sharing of electrons between atoms.
B)the transfer of electrons.
C)a bond between a metal and a nonmetal.
D)a bond between a metal and a polyatomic ion.
E)a bond between two polyatomic ions.
A)the sharing of electrons between atoms.
B)the transfer of electrons.
C)a bond between a metal and a nonmetal.
D)a bond between a metal and a polyatomic ion.
E)a bond between two polyatomic ions.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following contains both ionic and covalent bonds?
A)KI
B)NH4Cl
C)CaS
D)H2S
E)Si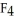
A)KI
B)NH4Cl
C)CaS
D)H2S
E)Si
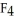

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
8
Identify the compound with (an)ionic bond(s).
A)C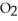
B)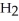
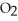
C)CH4
D)LiBr
E)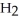
A)C
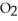
B)
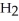
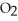
C)CH4
D)LiBr
E)
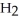

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is a molecular element?
A)Kr
B)Ag
C)S
D)Mg
E)Ti
A)Kr
B)Ag
C)S
D)Mg
E)Ti

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is an ionic compound?
A)LiCl
B)NO2
C)PCl3
D)CF4
E)SeBr2
A)LiCl
B)NO2
C)PCl3
D)CF4
E)SeBr2

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
11
An ionic bond is best described as
A)the sharing of electrons.
B)the transfer of electrons from one atom to another.
C)the attraction that holds the atoms together in a polyatomic ion.
D)the attraction between two nonmetal atoms.
E)the attraction between two metal atoms.
A)the sharing of electrons.
B)the transfer of electrons from one atom to another.
C)the attraction that holds the atoms together in a polyatomic ion.
D)the attraction between two nonmetal atoms.
E)the attraction between two metal atoms.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
12
Identify the compound with (a)covalent bond(s).
A)CH4
B)CaCl2
C)KBr
D)MgCl2
E)NaCl
A)CH4
B)CaCl2
C)KBr
D)MgCl2
E)NaCl

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is a molecular compound?
A)CuCl2
B)KCl
C)NaNO3
D)CH3Cl
E)RbBr
A)CuCl2
B)KCl
C)NaNO3
D)CH3Cl
E)RbBr

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
14
When forming ionic compounds,cations and anions form three-dimensional networks known as
A)a molecular compound.
B)a space-filling compound.
C)a lattice.
D)an empirical formula.
A)a molecular compound.
B)a space-filling compound.
C)a lattice.
D)an empirical formula.

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is an ionic compound?
A)SCl2
B)Mg3(PO4)2
C)Cl2O
D)CH2O
E)PF5
A)SCl2
B)Mg3(PO4)2
C)Cl2O
D)CH2O
E)PF5

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
16
Identify the compound with (an)ionic bond(s).
A)SO2
B)CO
C)O2
D)H2O
E)KBr
A)SO2
B)CO
C)O2
D)H2O
E)KBr

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following exists as a polyatomic molecule?
A)He
B)C
C)P
D)Na
E)Ne
A)He
B)C
C)P
D)Na
E)Ne

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is a molecular compound?
A)NaCN
B)LiOH
C)SrI2
D)ZnS
E)P4O10
A)NaCN
B)LiOH
C)SrI2
D)ZnS
E)P4O10

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following exists as a diatomic molecule?
A)N
B)C
C)P
D)Na
E)Ne
A)N
B)C
C)P
D)Na
E)Ne

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is a possible molecular formula for a compound with an empirical formula C4H4O?
A)C8H8O2
B)C12H12O2
C)C2H2O
D)C8H8O
A)C8H8O2
B)C12H12O2
C)C2H2O
D)C8H8O

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is an acid in aqueous solution?
A)NH3
B)NaF
C)Ca(C2H3O2)2
D)HI
E)KCl
A)NH3
B)NaF
C)Ca(C2H3O2)2
D)HI
E)KCl

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
22
Identify the charge,X,on the acetate ion .
A)+1
B)+2
C)+3
D)-2
E)-1
A)+1
B)+2
C)+3
D)-2
E)-1

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
23
Identify the formula for strontium nitride.
A)Sr3N2
B)Sr(NO3)2
C)SrN
D)Sr2N3
E)Sr(NO2)2
A)Sr3N2
B)Sr(NO3)2
C)SrN
D)Sr2N3
E)Sr(NO2)2

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
24
Identify the charge,X,on the phosphide ion PX.
A)-4
B)-3
C)-2
D)-1
E)+1
A)-4
B)-3
C)-2
D)-1
E)+1

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
25
Identify the name for LiNO3∙3H2O.
A)lithium nitrate
B)lithium nitrate hemihydrate
C)lithium nitrate monohydrate
D)lithium nitrate dihydrate
E)lithium nitrate trihydrate
A)lithium nitrate
B)lithium nitrate hemihydrate
C)lithium nitrate monohydrate
D)lithium nitrate dihydrate
E)lithium nitrate trihydrate

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is a molecular compound?
A)CuCl2
B)N2O4
C)NaNO3
D)SrSO3
E)RbBr
A)CuCl2
B)N2O4
C)NaNO3
D)SrSO3
E)RbBr

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
27
Determine the name for TiCO3.Remember that titanium forms several ions.
A)titanium(II)carbonate
B)titanium carbide
C)titanium carbonite
D)titanium(II)carbonite
E)titanium(I)carbonate
A)titanium(II)carbonate
B)titanium carbide
C)titanium carbonite
D)titanium(II)carbonite
E)titanium(I)carbonate

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
28
Identify the sulfite ion.
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
29
Identify the formula for magnesium nitrate hexahydrate.
A)Mg(NO3)2∙5H2O
B)Mg(NO3)2∙6H2O
C)Mg(NO3)2∙7H2O
D)MgNO3∙5H2O
E)MgNO3∙6H2O
A)Mg(NO3)2∙5H2O
B)Mg(NO3)2∙6H2O
C)Mg(NO3)2∙7H2O
D)MgNO3∙5H2O
E)MgNO3∙6H2O

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
30
Identify the cyanide ion.
A)
B)H
C)
D)HCN
E)
A)
B)H
C)
D)HCN
E)

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
31
Identify the perchlorate ion.
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
32
Identify the formula for sodium chlorate.
A)NaClO
B)NaClO2
C)NaClO3
D)NaClO4
A)NaClO
B)NaClO2
C)NaClO3
D)NaClO4

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is an acid in aqueous solution?
A)CaCO3
B)HClO2
C)CH3OCH3
D)NaCl
E)KNO3
A)CaCO3
B)HClO2
C)CH3OCH3
D)NaCl
E)KNO3

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
34
Identify the nitrite ion.
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
35
What is the empirical formula for C4H10O2?
A)C2H5O
B)CHO
C)C2H4O
D)CHO2
E)CH2O
A)C2H5O
B)CHO
C)C2H4O
D)CHO2
E)CH2O

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
36
Identify the formula for the compound formed between potassium and sulfur.
A)KS
B)KS2
C)K2S
D)K2SO3
E)K3S2
A)KS
B)KS2
C)K2S
D)K2SO3
E)K3S2

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
37
Identify the charge,X,on the barium ion .
A)+1
B)+2
C)+3
D)-1
E)-2
A)+1
B)+2
C)+3
D)-1
E)-2

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
38
Identify the name for SnO.
A)tin(I)oxide
B)tin(II)oxide
C)tin(III)oxide
D)tin(IV)oxide
A)tin(I)oxide
B)tin(II)oxide
C)tin(III)oxide
D)tin(IV)oxide

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
39
Identify the formula for barium hydroxide octahydrate.
A)Ba(OH)2∙8H2O
B)BaOH∙8H2O
C)B(OH)3∙8H2O
D)8Ba(OH)2∙H2O
E)8BaOH∙H2O
A)Ba(OH)2∙8H2O
B)BaOH∙8H2O
C)B(OH)3∙8H2O
D)8Ba(OH)2∙H2O
E)8BaOH∙H2O

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
40
Identify the formula for barium nitrite.
A)Ba3N2
B)BaNO3
C)BN
D)Ba(NO2)2
E)B(NO2)3
A)Ba3N2
B)BaNO3
C)BN
D)Ba(NO2)2
E)B(NO2)3

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
41
Determine the name for H2CO3.
A)carbonous acid
B)dihydrogen carbonate
C)carbonic acid
D)hydrocarbonic acid
E)hydrocarbide acid
A)carbonous acid
B)dihydrogen carbonate
C)carbonic acid
D)hydrocarbonic acid
E)hydrocarbide acid

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
42
Determine the name for CoCl2∙6H2O.Remember that Co forms several ions.
A)cobalt chloride hydrate
B)cobalt(I)chloride heptahydrate
C)cobalt(II)chloride heptahydrate
D)cobalt(II)chloride hexahydrate
E)cobalt(I)chloride
A)cobalt chloride hydrate
B)cobalt(I)chloride heptahydrate
C)cobalt(II)chloride heptahydrate
D)cobalt(II)chloride hexahydrate
E)cobalt(I)chloride

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
43
Identify the name for aqueous H2SO4.
A)sulfuric acid
B)persulfurous acid
C)sulfurous acid
D)hyposulfurous acid
E)persulfuric acid
A)sulfuric acid
B)persulfurous acid
C)sulfurous acid
D)hyposulfurous acid
E)persulfuric acid

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
44
Determine the name for Cl2O.
A)chlorine oxide
B)dichlorine monoxide
C)chlorine(I)oxide
D)chlorine(II)oxide
E)chlorate
A)chlorine oxide
B)dichlorine monoxide
C)chlorine(I)oxide
D)chlorine(II)oxide
E)chlorate

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
45
Identify the name for FeS.
A)iron(I)sulfate
B)iron(I)sulfide
C)iron(II)sulfide
D)iron(II)sulfate
E)iron sulfide
A)iron(I)sulfate
B)iron(I)sulfide
C)iron(II)sulfide
D)iron(II)sulfate
E)iron sulfide

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
46
Identify the name for Sr(NO3)2∙4H2O.
A)tin(II)nitrate tetrahydrate
B)strontium nitrate tetrahydrate
C)scandium nitrate tetrahydrate
D)tin(III)nitrate tetrahydrate
E)tin(II)nitrate quadhydrate
A)tin(II)nitrate tetrahydrate
B)strontium nitrate tetrahydrate
C)scandium nitrate tetrahydrate
D)tin(III)nitrate tetrahydrate
E)tin(II)nitrate quadhydrate

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
47
Determine the name for aqueous HBr.
A)bromic acid
B)bromous acid
C)hydrobromous acid
D)hydrogen bromate
E)hydrobromic acid
A)bromic acid
B)bromous acid
C)hydrobromous acid
D)hydrogen bromate
E)hydrobromic acid

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
48
Identify the name for Sn(SO4)2.Remember that Sn forms several ions.
A)tin(I)sulfite
B)tin(IV)sulfate
C)tin sulfide
D)tin(II)sulfite
E)tin(I)sulfate
A)tin(I)sulfite
B)tin(IV)sulfate
C)tin sulfide
D)tin(II)sulfite
E)tin(I)sulfate

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
49
What is the charge on an aluminum ion?
A)+3
B)+2
C)+1
D)-1
E)-2
A)+3
B)+2
C)+1
D)-1
E)-2

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
50
What is the correct formula for lead (II)chloride?
A)LdCl4
B)SbCl4
C)PbCl2
D)PbCl4
E)LaCl3
A)LdCl4
B)SbCl4
C)PbCl2
D)PbCl4
E)LaCl3

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
51
Determine the name for N2O5.
A)dinitrogen pentoxide
B)nitrogen oxide
C)nitrogen(IV)oxide
D)nitrogen(II)oxide
E)nitrogen tetroxide
A)dinitrogen pentoxide
B)nitrogen oxide
C)nitrogen(IV)oxide
D)nitrogen(II)oxide
E)nitrogen tetroxide

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
52
Identify the correct formula for sodium chlorate.
A)NaClO
B)NaClO2
C)NaClO3
D)NaClO4
A)NaClO
B)NaClO2
C)NaClO3
D)NaClO4

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
53
Identify the correct formula for aluminum sulfate.
A)Al2SO4
B)Al(SO4)3
C)Al3(SO4)2
D)Al2(SO4)3
A)Al2SO4
B)Al(SO4)3
C)Al3(SO4)2
D)Al2(SO4)3

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
54
Determine the name for aqueous HClO3.
A)hydrochloric acid
B)hydrochlorous acid
C)chlorate acid
D)chloric acid
E)perchloric acid
A)hydrochloric acid
B)hydrochlorous acid
C)chlorate acid
D)chloric acid
E)perchloric acid

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
55
Determine the name for P4O10.
A)phosphorus(IV)oxide
B)diphosphorus pentoxide
C)phosphorus oxide
D)phosphorus(II)oxide
E)tetraphosphorus decoxide
A)phosphorus(IV)oxide
B)diphosphorus pentoxide
C)phosphorus oxide
D)phosphorus(II)oxide
E)tetraphosphorus decoxide

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
56
Identify the name for Ca3(PO4)2.
A)calcium(III)phosphite
B)calcium(II)phosphite
C)calcium phosphate
D)tricalcium phosphorustetraoxide
E)calcium phosphite
A)calcium(III)phosphite
B)calcium(II)phosphite
C)calcium phosphate
D)tricalcium phosphorustetraoxide
E)calcium phosphite

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
57
Identify the name for PBr3.
A)phosphorus tribromide
B)potassium tribromide
C)phosphorus(III)bromide
D)phosphorus(II)bromide
E)phosphorus bromide
A)phosphorus tribromide
B)potassium tribromide
C)phosphorus(III)bromide
D)phosphorus(II)bromide
E)phosphorus bromide

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
58
Identify the formula for copper (II)sulfate pentahydrate.
A)Cu2SO3∙H5
B)Cu2S∙H2O
C)CuS∙5H2O
D)(CuSO4)5
E)CuSO4∙5H2O
A)Cu2SO3∙H5
B)Cu2S∙H2O
C)CuS∙5H2O
D)(CuSO4)5
E)CuSO4∙5H2O

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
59
Identify the formula for sulfurous acid.
A)H2SO3
B)HSO3
C)H2SO4
D)HSO4
A)H2SO3
B)HSO3
C)H2SO4
D)HSO4

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
60
Identify the formula for chromium(II)nitrate.
A)Cr(NO2)3
B)Cr(NO3)3
C)Cr(NO3)2
D)Cr(NO2)2
E)Cr2NO2
A)Cr(NO2)3
B)Cr(NO3)3
C)Cr(NO3)2
D)Cr(NO2)2
E)Cr2NO2

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following is a formula for the compound disulfur tetrafluoride?
A)SF4
B)2SF4
C)S2F4
D)SF6
E)S2F2
A)SF4
B)2SF4
C)S2F4
D)SF6
E)S2F2

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
62
Calculate the molar mass of H2CO3.
A)62.03 g mol-1
B)29.02 g mol-1
C)61.02 g mol-1
D)60.01 g mol-1
E)74.04 g mol-1
A)62.03 g mol-1
B)29.02 g mol-1
C)61.02 g mol-1
D)60.01 g mol-1
E)74.04 g mol-1

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following is a formula for the compound iridium(III)bromide tetrahydrate?
A)IrBr∙ H2O
H2O
B)IrBr∙4H2O
C)IrBr3∙4H2O
D)IBr3∙4H2O
E)IBr3∙2H2O
A)IrBr∙
 H2O
H2OB)IrBr∙4H2O
C)IrBr3∙4H2O
D)IBr3∙4H2O
E)IBr3∙2H2O

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
64
Identify the name for the compound Fe(C2H3O2)2.
A)iron(II)hydrogen carbonate
B)iron(III)carbonate
C)iron acetate
D)iron(II)acetate
E)iron(II)dihydrogen phosphate
A)iron(II)hydrogen carbonate
B)iron(III)carbonate
C)iron acetate
D)iron(II)acetate
E)iron(II)dihydrogen phosphate

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
65
Calculate the molar mass for Mg(ClO4)2.
A)223.21 g mol-1
B)123.76 g mol-1
C)119.52 g mol-1
D)247.52 g mol-1
E)75.76 g mol-1
A)223.21 g mol-1
B)123.76 g mol-1
C)119.52 g mol-1
D)247.52 g mol-1
E)75.76 g mol-1

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
66
Identify an amine.
A)CH3CH2OCH2CH3
B)CH3CH2OH
C)CH3CH2NH2
D)CH3CH2CH2CH3
E)CH3COOH
A)CH3CH2OCH2CH3
B)CH3CH2OH
C)CH3CH2NH2
D)CH3CH2CH2CH3
E)CH3COOH

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
67
How many molecules of N2O4 are in 76.3 g N2O4? The molar mass of N2O4 is 92.02 g mol-1.
A)5.54 × 1025 N2O4 molecules
B)7.26 × 1023 N2O4 molecules
C)1.38 × 1024 N2O4 molecules
D)4.59 × 1025 N2O4 molecules
E)4.99 × 1023 N2O4 molecules
A)5.54 × 1025 N2O4 molecules
B)7.26 × 1023 N2O4 molecules
C)1.38 × 1024 N2O4 molecules
D)4.59 × 1025 N2O4 molecules
E)4.99 × 1023 N2O4 molecules

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
68
Identify an ether.
A)CH3CH2OCH2CH3
B)CH3CH2OH
C)CH3CH2NH2
D)CH3CH2CH2CH3
E)CH3COOH
A)CH3CH2OCH2CH3
B)CH3CH2OH
C)CH3CH2NH2
D)CH3CH2CH2CH3
E)CH3COOH

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
69
How many C2H4 molecules are contained in 45.8 mg of C2H4? The molar mass of C2H4 is 28.05 g mol-1.
A)9.83 × 1020 C2H4 molecules
B)7.74 × 1026 C2H4 molecules
C)2.71 × 1020 C2H4 molecules
D)3.69 × 1023 C2H4 molecules
E)4.69 × 1023 C2H4 molecules
A)9.83 × 1020 C2H4 molecules
B)7.74 × 1026 C2H4 molecules
C)2.71 × 1020 C2H4 molecules
D)3.69 × 1023 C2H4 molecules
E)4.69 × 1023 C2H4 molecules

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
70
Calculate the molar mass of Al(C2H3O2)3.
A)86.03 g mol-1
B)204.13 g mol-1
C)56.00 g mol-1
D)258.09 g mol-1
E)139.99 g mol-1
A)86.03 g mol-1
B)204.13 g mol-1
C)56.00 g mol-1
D)258.09 g mol-1
E)139.99 g mol-1

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
71
Identify a hydrocarbon.
A)CH3CH2OCH2CH3
B)CH3CH2OH
C)CH3CH2NH2
D)CH3CH2CH2CH3
E)CH3COOH
A)CH3CH2OCH2CH3
B)CH3CH2OH
C)CH3CH2NH2
D)CH3CH2CH2CH3
E)CH3COOH

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
72
How many millimoles of Ca(NO3)2 contain 4.78 × 1022 formula units of Ca(NO3)2? The molar mass of Ca(NO3)2 is 164.10 g mol-1.
A)12.6 mmol Ca(NO3)2
B)13.0 mmol Ca(NO3)2
C)20.7 mmol Ca(NO3)2
D)79.4 mmol Ca(NO3)2
E)57.0 mmol Ca(NO3)2
A)12.6 mmol Ca(NO3)2
B)13.0 mmol Ca(NO3)2
C)20.7 mmol Ca(NO3)2
D)79.4 mmol Ca(NO3)2
E)57.0 mmol Ca(NO3)2

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
73
Identify a carboxylic acid.
A)CH3CH2OCH2CH3
B)CH3CH2OH
C)CH3CH2NH2
D)CH3CH2CH2CH3
E)CH3COOH
A)CH3CH2OCH2CH3
B)CH3CH2OH
C)CH3CH2NH2
D)CH3CH2CH2CH3
E)CH3COOH

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
74
What is the mass (in kg)of 6.89 × 1025 molecules of CO2? The molar mass of CO2 is 44.01 g mol-1.
A)3.85 kg
B)5.04 kg
C)2.60 kg
D)3.03 kg
E)6.39 kg
A)3.85 kg
B)5.04 kg
C)2.60 kg
D)3.03 kg
E)6.39 kg

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
75
What is the mass of 9.44 × 1024 molecules of NO2? The molar mass of NO2 is 46.01 g mol-1.
A)205 g
B)294 g
C)721 g
D)341 g
E)685 g
A)205 g
B)294 g
C)721 g
D)341 g
E)685 g

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
76
How many moles of C3H8 contain 9.25 × 1024 molecules of C3H8?
A)65.1 moles C3H8
B)28.6 moles C3H8
C)34.9 moles C3H8
D)46.2 moles C3H8
E)15.4 moles C3H8
A)65.1 moles C3H8
B)28.6 moles C3H8
C)34.9 moles C3H8
D)46.2 moles C3H8
E)15.4 moles C3H8

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
77
How many moles of N2O4 are in 76.3 g N2O4? The molar mass of N2O4 is 92.02 g mol-1.
A)7.02 × 103 moles N2O4
B)1.42 × 10-4 moles N2O4
C)1.00 mole N2O4
D)1.21 moles N2O4
E)0.829 moles N2O4
A)7.02 × 103 moles N2O4
B)1.42 × 10-4 moles N2O4
C)1.00 mole N2O4
D)1.21 moles N2O4
E)0.829 moles N2O4

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following compounds is ethanol?
A)C2H6
B)C2H5OH
C)CH3CO2H
D)CH3CO2CH3
E)CH3-O-CH3
A)C2H6
B)C2H5OH
C)CH3CO2H
D)CH3CO2CH3
E)CH3-O-CH3

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
79
Calculate the molar mass of Ca3(PO4)2.
A)87.05 g mol-1
B)215.21 g mol-1
C)310.18 g mol-1
D)279.21 g mol-1
E)246.18 g mol-1
A)87.05 g mol-1
B)215.21 g mol-1
C)310.18 g mol-1
D)279.21 g mol-1
E)246.18 g mol-1

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following is one possible form of pentane?
A)CH3CH2CH2CH2CH3
B)CH3CH=CHCH2CH3
C)CH3CH2CH2CH2CH2CH3
D)CH3CH2CH2CH2CH2NH2
E)CH3CH2-O-CH2CH2CH3
A)CH3CH2CH2CH2CH3
B)CH3CH=CHCH2CH3
C)CH3CH2CH2CH2CH2CH3
D)CH3CH2CH2CH2CH2NH2
E)CH3CH2-O-CH2CH2CH3

Unlock Deck
Unlock for access to all 172 flashcards in this deck.
Unlock Deck
k this deck



