Deck 5: Chemical Bonding and Nomenclature
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/260
Play
Full screen (f)
Deck 5: Chemical Bonding and Nomenclature
1
How many valence electrons are needed to construct the Lewis dot structure of the BCl3 molecule?
A)11
B)21
C)24
D)32
A)11
B)21
C)24
D)32
24
2
What is the chemical formula of rhenium(VII)oxide?
A)ReO7
B)ReO4
C)Re2O7
D)Re3O6
A)ReO7
B)ReO4
C)Re2O7
D)Re3O6
Re2O7
3
Which of the following covalent bonds would you expect to be the least polar?
A)C-C
B)C-H
C)C-F
D)C-Cl
A)C-C
B)C-H
C)C-F
D)C-Cl
C-C
4
How many valence electrons does a single, neutral iodine atom have?
A)3
B)4
C)5
D)7
A)3
B)4
C)5
D)7

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
5
How many covalent bonds can an element of Group VA form with other elements?
A)4
B)3
C)2
D)1
A)4
B)3
C)2
D)1

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements best describes an ionic bond?
A)a strong lattice of positively and negatively charged atoms held together by electrical forces
B)the bonding type present in bound, non-metal atoms
C)the equal sharing of two electrons between adjacent atoms
D)the bonding type present between two metal atoms
A)a strong lattice of positively and negatively charged atoms held together by electrical forces
B)the bonding type present in bound, non-metal atoms
C)the equal sharing of two electrons between adjacent atoms
D)the bonding type present between two metal atoms

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following represents the correct Lewis dot structure for selenium (Se)?
A)
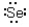
B)
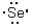
C)

D)
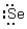
A)
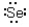
B)
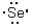
C)

D)
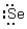

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
8
What is the chemical formula of calcium phosphate?
A)CaPO4
B)Ca2PO4
C)Ca3PO4
D)Ca3(PO4)2
A)CaPO4
B)Ca2PO4
C)Ca3PO4
D)Ca3(PO4)2

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following substances is molecular (composed of individual molecules)in composition?
A)carbon, as coal
B)water, as a glass of water
C)copper, as an ingot
D)helium, as found in a balloon
A)carbon, as coal
B)water, as a glass of water
C)copper, as an ingot
D)helium, as found in a balloon

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following covalent bonds would you expect to be the most polar?
A)C-C
B)C-H
C)C-F
D)C-Cl
A)C-C
B)C-H
C)C-F
D)C-Cl

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is considered an elemental substance?
A)I2 (iodine)
B)H2O (water)
C)Fe (an iron nail)
D)A and C are elemental substances.
E)All of the preceding are elemental substances.
A)I2 (iodine)
B)H2O (water)
C)Fe (an iron nail)
D)A and C are elemental substances.
E)All of the preceding are elemental substances.

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
12
What is the most likely formula of the neutral, ionic compound formed by lithium and nitrogen?
A)Li2N
B)Li3N
C)LiN
D)LiN2
A)Li2N
B)Li3N
C)LiN
D)LiN2

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following bonds would you expect to be ionic?
A)C-S
B)Na-F
C)N-O
D)F-F
A)C-S
B)Na-F
C)N-O
D)F-F

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
14
To follow the octet rule, how many electrons must a neutral sulfur atom gain when forming a negative ion?
A)1
B)2
C)6
D)8
A)1
B)2
C)6
D)8

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
15
How many covalent bonds can a single oxygen atom form with other elements?
A)3
B)4
C)2
D)1
A)3
B)4
C)2
D)1

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following phrases best describes the H-O bonds present in water (H2O)?
A)nonpolar and ionic
B)nonpolar and covalent
C)polar and ionic
D)polar and covalent
A)nonpolar and ionic
B)nonpolar and covalent
C)polar and ionic
D)polar and covalent

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
17
How many unpaired electrons does the oxide anion (O2-)have?
A)3
B)2
C)1
D)0
A)3
B)2
C)1
D)0

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
18
What is the most likely formula of the neutral, ionic compound formed by magnesium and oxygen?
A)Mg2O
B)MgO2
C)Mg3O2
D)MgO
A)Mg2O
B)MgO2
C)Mg3O2
D)MgO

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
19
What is the chemical name of the compound whose formula is K2O?
A)dipotassium oxygen
B)potassium oxide
C)potasside oxygen
D)potassium oxygen
A)dipotassium oxygen
B)potassium oxide
C)potasside oxygen
D)potassium oxygen

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
20
Which phrase best describes a covalent bond between two atoms?
A)a stable arrangement of charged atoms held together by electrostatic forces
B)a stable arrangement of atoms made by sharing two electrons between adjacent atoms
C)uncommon, as electrons are free to jump from one atom to another
D)an exchange of nuclei between two atoms
A)a stable arrangement of charged atoms held together by electrostatic forces
B)a stable arrangement of atoms made by sharing two electrons between adjacent atoms
C)uncommon, as electrons are free to jump from one atom to another
D)an exchange of nuclei between two atoms

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
21
A binary covalent compound is composed of ________.
A)two metalloids
B)a metal and a non-metal
C)two non-metals
D)either A or C
A)two metalloids
B)a metal and a non-metal
C)two non-metals
D)either A or C

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
22
Every single COVALENT bond represents ________ electrons.
A)one
B)two
C)four
D)One cannot say unless the atoms that make up the bond are known.
A)one
B)two
C)four
D)One cannot say unless the atoms that make up the bond are known.

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
23
If the electrons are shared equally between the two atoms, the bond is called ________.
A)ionic
B)non-polar covalent
C)hydrogen bonding
D)polar covalent
A)ionic
B)non-polar covalent
C)hydrogen bonding
D)polar covalent

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is a binary compound?
A)sodium perchlorate
B)sodium chlorate
C)sodium hypochlorite
D)sodium chloride
A)sodium perchlorate
B)sodium chlorate
C)sodium hypochlorite
D)sodium chloride

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
25
When a binary compound is composed of a metal and a non-metal it is called ________.
A)binary ionic
B)binary covalent
C)binary coordinate
D)either B or C
A)binary ionic
B)binary covalent
C)binary coordinate
D)either B or C

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following compounds has a covalent bond?
A)NaCl
B)KBr
C)LiH
D)H2
A)NaCl
B)KBr
C)LiH
D)H2

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following sets is comprised exclusively of ionic binary compounds?
A)H2O, SiO2, NO
B)CoCl2, CaCO3, AlPO4
C)Co(H2PO4)2, KCl, PCl3
D)KI, Al2O3, Li2S
A)H2O, SiO2, NO
B)CoCl2, CaCO3, AlPO4
C)Co(H2PO4)2, KCl, PCl3
D)KI, Al2O3, Li2S

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
28
When electrons in a bond are shared unequally the bond is ________.
A)ionic
B)non-polar covalent
C)hydrogen bonding
D)polar covalent
A)ionic
B)non-polar covalent
C)hydrogen bonding
D)polar covalent

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is not a binary compound?
A)sodium chloride
B)sodium hypochlorite
C)sodium sulfide
D)sodium hydride
A)sodium chloride
B)sodium hypochlorite
C)sodium sulfide
D)sodium hydride

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
30
Which is not a binary covalent compound?
A)CO
B)H2O
C)CaF2
D)SiO2
A)CO
B)H2O
C)CaF2
D)SiO2

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
31
The numerical rating of an atom's ability to attract the shared electrons is called ________.
A)electronegativity
B)ionization
C)covalency
D)redistribution
A)electronegativity
B)ionization
C)covalency
D)redistribution

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following sets is comprised exclusively of covalent binary compounds?
A)H2O, SiO2, NO
B)CoCl2, CaCO3, AlPO4
C)Co(H2PO4)2, KCl, PCl3
D)KI, Al2O3, Li2S
A)H2O, SiO2, NO
B)CoCl2, CaCO3, AlPO4
C)Co(H2PO4)2, KCl, PCl3
D)KI, Al2O3, Li2S

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
33
When the valence electrons between atoms are transferred the bond is called ________.
A)ionic
B)covalent
C)hydrogen bonding
D)polar covalent
A)ionic
B)covalent
C)hydrogen bonding
D)polar covalent

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
34
What is the chemical formula of a compound named ammonium sulfide?
A)NH4S
B)(NH4)2S
C)(NH4)S2
D)(NH4)2S3
A)NH4S
B)(NH4)2S
C)(NH4)S2
D)(NH4)2S3

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
35
The number of valence electrons in silicon is ________.
A)2
B)4
C)6
D)8
A)2
B)4
C)6
D)8

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
36
What is the chemical name of the compound whose formula is MgSO4?
A)There is no such compound.
B)magnesium sulfur tetraoxide
C)magnesium sulfide
D)magnesium sulfate
A)There is no such compound.
B)magnesium sulfur tetraoxide
C)magnesium sulfide
D)magnesium sulfate

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
37
What is the chemical formula of the compound named potassium bicarbonate?
A)KHCO3
B)K2(HCO3)
C)K(HCO3)2
D)K3HCO3
A)KHCO3
B)K2(HCO3)
C)K(HCO3)2
D)K3HCO3

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is a binary ionic compound?
A)potassium sulfide
B)phosphorus trichloride
C)sulfur hexafluoride
D)water
A)potassium sulfide
B)phosphorus trichloride
C)sulfur hexafluoride
D)water

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is not a binary ionic compound?
A)lithium sulfide
B)potassium iodide
C)calcium chloride
D)hydrogen sulfide
A)lithium sulfide
B)potassium iodide
C)calcium chloride
D)hydrogen sulfide

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
40
What is the chemical name given to the compound whose formula is N2O4?
A)nitrogen quadraoxide
B)nitrogen (IV)oxide
C)nitrogen (II)oxygen (IV)
D)dinitrogen tetraoxide
A)nitrogen quadraoxide
B)nitrogen (IV)oxide
C)nitrogen (II)oxygen (IV)
D)dinitrogen tetraoxide

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following elements has the lowest number of valence electrons?
A)magnesium
B)lithium
C)argon
D)phosphorus
A)magnesium
B)lithium
C)argon
D)phosphorus

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
42
The expected formula between silicon and fluorine is ________.
A)SiF
B)SiF2
C)SiF4
D)SiF6
A)SiF
B)SiF2
C)SiF4
D)SiF6

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following pairs has the same number valence electrons?
A)magnesium and potassium
B)lithium and calcium
C)argon and xenon
D)phosphorus and tellurium
A)magnesium and potassium
B)lithium and calcium
C)argon and xenon
D)phosphorus and tellurium

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
44
The expected binary compound between magnesium and bromine is ________.
A)MgBr
B)MgBr2
C)Mg2Br
D)Mg2Br2
A)MgBr
B)MgBr2
C)Mg2Br
D)Mg2Br2

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following has the same number of valence electrons as sulfur?
A)nitrogen
B)oxygen
C)arsenic
D)boron
A)nitrogen
B)oxygen
C)arsenic
D)boron

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
46
The number of valence electrons in sulfur is ________.
A)2
B)4
C)6
D)8
A)2
B)4
C)6
D)8

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following elements has the highest number of valence electrons?
A)magnesium
B)lithium
C)argon
D)phosphorus
A)magnesium
B)lithium
C)argon
D)phosphorus

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
48
The expected formula between calcium and oxygen is ________.
A)CaO
B)CaO2
C)Ca2O
D)Ca2O5
A)CaO
B)CaO2
C)Ca2O
D)Ca2O5

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
49
The number of valence electrons in aluminum is ________.
A)2
B)3
C)5
D)7
A)2
B)3
C)5
D)7

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
50
The number of valence electrons in nitrogen is ________.
A)2
B)3
C)5
D)6
A)2
B)3
C)5
D)6

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is the least electronegative?
A)fluorine
B)oxygen
C)carbon
D)sodium
A)fluorine
B)oxygen
C)carbon
D)sodium

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
52
The expected formula between calcium and iodine is ________.
A)CaI
B)Cal2
C)Ca2I
D)Ca2I3
A)CaI
B)Cal2
C)Ca2I
D)Ca2I3

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
53
The expected formula between magnesium and sulfur is ________.
A)MgS
B)MgS2
C)Mg2S
D)Mg2S3
A)MgS
B)MgS2
C)Mg2S
D)Mg2S3

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
54
The expected formula between sodium and oxygen is ________.
A)NaO
B)Na2O
C)NaO2
D)Na2O3
A)NaO
B)Na2O
C)NaO2
D)Na2O3

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
55
The expected formula between aluminum and oxygen is ________.
A)AlO
B)AlO2
C)Al2O
D)Al2O3
A)AlO
B)AlO2
C)Al2O
D)Al2O3

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
56
The number of valence electrons in oxygen is ________.
A)2
B)4
C)6
D)8
A)2
B)4
C)6
D)8

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following elements has the least number of valence electrons?
A)magnesium
B)lithium
C)argon
D)phosphorus
A)magnesium
B)lithium
C)argon
D)phosphorus

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is the most electronegative?
A)fluorine
B)oxygen
C)carbon
D)sodium
A)fluorine
B)oxygen
C)carbon
D)sodium

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following does not have the same number of valence electrons as the other three elements?
A)nitrogen
B)phosphorus
C)beryllium
D)arsenic
A)nitrogen
B)phosphorus
C)beryllium
D)arsenic

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
60
The number of valence electrons in neon is ________.
A)2
B)4
C)6
D)8
A)2
B)4
C)6
D)8

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following may not exist as a diatomic molecule?
A)oxygen
B)nitrogen
C)argon
D)hydrogen
A)oxygen
B)nitrogen
C)argon
D)hydrogen

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
62
Nitrogen trifluoride has ________ bonds, ________ of which are single bonds.
A)3; 3
B)5; 1
C)7; 2
D)8; 3
A)3; 3
B)5; 1
C)7; 2
D)8; 3

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
63
Phosphine, PH3, has ________ covalent pairs and ________ lone pairs.
A)3; 0
B)3; 1
C)3; 2
D)2; 0
A)3; 0
B)3; 1
C)3; 2
D)2; 0

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
64
What is the number of single bonds in C2H6O?
A)5
B)6
C)7
D)8
A)5
B)6
C)7
D)8

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
65
Each atom in the oxygen molecule contributes ________ valence electrons for a total of ________ electrons
A)6; 12
B)6; 8
C)8; 6
D)12; 6
A)6; 12
B)6; 8
C)8; 6
D)12; 6

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
66
Methane has ________ covalent bonds and ________ lone pairs.
A)4; 2
B)4; 1
C)4; 0
D)2; 4
A)4; 2
B)4; 1
C)4; 0
D)2; 4

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
67
Hydrazine, N2H4, has ________ covalent bonds and ________ lone pairs.
A)2; 2
B)4; 2
C)3; 4
D)5; 2
A)2; 2
B)4; 2
C)3; 4
D)5; 2

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
68
The number of valence electrons in the nitrate anion is ________.
A)23
B)24
C)28
D)30
A)23
B)24
C)28
D)30

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
69
Nitrogen trifluoride has ________ valence electrons.
A)20
B)22
C)24
D)26
A)20
B)22
C)24
D)26

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
70
Hydrazine has ________ valence electrons.
A)14
B)12
C)10
D)8
A)14
B)12
C)10
D)8

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
71
The expected formula between hydrogen and nitrogen is ________.
A)HN
B)NH2
C)NH3
D)NH4
A)HN
B)NH2
C)NH3
D)NH4

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
72
Acetylene, C2H2, has ________.
A)two single bonds
B)two double bonds
C)one triple bond
D)both A and C
A)two single bonds
B)two double bonds
C)one triple bond
D)both A and C

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
73
The number of valence electrons that belong to the oxygens in the carbonate anion is ________.
A)15
B)18
C)20
D)24
A)15
B)18
C)20
D)24

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
74
The central atom in sulfuric acid is ________.
A)oxygen
B)sulfur
C)hydrogen
D)none of the above
A)oxygen
B)sulfur
C)hydrogen
D)none of the above

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
75
Water has ________ covalent bonds and ________ lone pairs.
A)2; 2
B)2; 4
C)2; 0
D)2; 6
A)2; 2
B)2; 4
C)2; 0
D)2; 6

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
76
Nitrogen trifluoride has ________ valence electrons that are contributed by the nitrogen atom.
A)5
B)8
C)10
D)12
A)5
B)8
C)10
D)12

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
77
The expected formula between potassium and sulfur is ________.
A)KS
B)K2S3
C)K2S
D)KS2
A)KS
B)K2S3
C)K2S
D)KS2

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
78
The number of valence electrons in the carbonate anion is ________.
A)20
B)22
C)24
D)26
A)20
B)22
C)24
D)26

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
79
The phosphate anion has ________ valence electrons.
A)29
B)30
C)32
D)36
A)29
B)30
C)32
D)36

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following exists as a diatomic molecule?
A)neon
B)oxygen
C)phosphorus
D)silicon
A)neon
B)oxygen
C)phosphorus
D)silicon

Unlock Deck
Unlock for access to all 260 flashcards in this deck.
Unlock Deck
k this deck



