Deck 8: Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns
Question
Question
Match between columns
Premises:
potassium nitrate
potassium nitrate
lead (II) sulfate
lead (II) sulfate
Responses:
water soluble
water insoluble
water soluble
water insoluble
water soluble
water insoluble
water soluble
water insoluble
water soluble
water insoluble
water soluble
water insoluble
water soluble
water insoluble
water soluble
water insoluble
water soluble
water insoluble
Question
Question
Match between columns
Question
Match between columns
Question
Match between columns
Premises:
H₂ + O₂ → H₂O
H₂ + O₂ → H₂O
H₂ + O₂ → H₂O
H₂ + O₂ → H₂O
H₂ + O₂ → H₂O
H₂ + O₂ → H₂O
NaClO₃ → NaCl + O₂
NaClO₃ → NaCl + O₂
NaClO₃ → NaCl + O₂
NaClO₃ → NaCl + O₂
NaClO₃ → NaCl + O₂
NaClO₃ → NaCl + O₂
CH₄ + O₂ → CO₂ + H₂O
CH₄ + O₂ → CO₂ + H₂O
CH₄ + O₂ → CO₂ + H₂O
CH₄ + O₂ → CO₂ + H₂O
CH₄ + O₂ → CO₂ + H₂O
CH₄ + O₂ → CO₂ + H₂O
Mg + HCl → H₂ + MgCl₂
Mg + HCl → H₂ + MgCl₂
Mg + HCl → H₂ + MgCl₂
Mg + HCl → H₂ + MgCl₂
Mg + HCl → H₂ + MgCl₂
Mg + HCl → H₂ + MgCl₂
Na₂SO₄ + PbCl₂ → PbSO₄
Na₂SO₄ + PbCl₂ → PbSO₄
Na₂SO₄ + PbCl₂ → PbSO₄
Na₂SO₄ + PbCl₂ → PbSO₄
Na₂SO₄ + PbCl₂ → PbSO₄
Na₂SO₄ + PbCl₂ → PbSO₄
H₂SO₄ + NaOH → Na₂SO₄ + H₂O
H₂SO₄ + NaOH → Na₂SO₄ + H₂O
H₂SO₄ + NaOH → Na₂SO₄ + H₂O
H₂SO₄ + NaOH → Na₂SO₄ + H₂O
H₂SO₄ + NaOH → Na₂SO₄ + H₂O
H₂SO₄ + NaOH → Na₂SO₄ + H₂O
Responses:
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/79
Play
Full screen (f)
Deck 8: Chemical Reactions
1
Which process absorbs energy?
A)bond formation
B)bond breaking
C)both A and B
D)none of the above
A)bond formation
B)bond breaking
C)both A and B
D)none of the above
bond breaking
2
The equation AgNO3 + NaCl → AgCl + NaNO3 is an example of a ________ reaction.
A)combination
B)precipitation
C)single replacement
D)decomposition
A)combination
B)precipitation
C)single replacement
D)decomposition
precipitation
3
In a gas-phase reaction ________.
A)only products are gases
B)only reactants are gases
C)both reactants and products are gases
D)solids and liquids may be present, but at least one reagent must be a gas
A)only products are gases
B)only reactants are gases
C)both reactants and products are gases
D)solids and liquids may be present, but at least one reagent must be a gas
only reactants are gases
4
The spectator ions in the precipitation equation Pb(NO3)2+ 2KI → PbI2 + 2KNO3 are ________.
A)Pb2+ and I-
B)K+ and NO3-
C)K+ and I-
D)Pb2+ and NO3-
A)Pb2+ and I-
B)K+ and NO3-
C)K+ and I-
D)Pb2+ and NO3-

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following reactions is a single replacement reaction?
A)HCl + NaOH → NaCl + H2O
B)Mg + HCl → H2 + MgCl2
C)CaCl2 + H2O → CaCl2 ∙ 2H2O
D)KClO3 → KCl + O2
A)HCl + NaOH → NaCl + H2O
B)Mg + HCl → H2 + MgCl2
C)CaCl2 + H2O → CaCl2 ∙ 2H2O
D)KClO3 → KCl + O2

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following equations is not a double replacement reaction?
A)H2SO4 + Mg(OH)2 → H2O + MgSO4
B)Mg + HCl → H2 + MgCl2
C)AlCl3 + H2O → Al(OH)3 + HCl
D)AgNO3 + NaCl → AgCl + NaNO3
A)H2SO4 + Mg(OH)2 → H2O + MgSO4
B)Mg + HCl → H2 + MgCl2
C)AlCl3 + H2O → Al(OH)3 + HCl
D)AgNO3 + NaCl → AgCl + NaNO3

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
7
The equation NaClO3 → NaCl + O2 is an example of a ________ reaction.
A)combination
B)combustion
C)decomposition
D)double replacement
A)combination
B)combustion
C)decomposition
D)double replacement

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
8
The equation Zn + CuCl2 → Cu + ZnCl2 is an example of a ________ reaction.
A)combination
B)single replacement
C)double replacement
D)decomposition
A)combination
B)single replacement
C)double replacement
D)decomposition

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
9
The equation Pb(NO3 )2+ 2KI → PbI2 + 2KNO3 is an example of a ________ reaction.
A)synthesis
B)precipitation
C)single replacement
D)decomposition
A)synthesis
B)precipitation
C)single replacement
D)decomposition

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following reactions is a decomposition reaction?
A)HCl + NaOH → NaCl + H2O
B)Mg + HCl → H2 + MgCl2
C)CaCl2 + H2O → CaCl2 ∙ 2H2O
D)KClO3 → KCl + O2
A)HCl + NaOH → NaCl + H2O
B)Mg + HCl → H2 + MgCl2
C)CaCl2 + H2O → CaCl2 ∙ 2H2O
D)KClO3 → KCl + O2

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following equations is not a combination reaction?
A)H2 + O2 → H2O
B)C2H4 + Br2 → C2H4Br2
C)CH4 + O2 → CO2 + H2O
D)Fe + O2 → Fe3O4
A)H2 + O2 → H2O
B)C2H4 + Br2 → C2H4Br2
C)CH4 + O2 → CO2 + H2O
D)Fe + O2 → Fe3O4

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following equations is a combustion reaction?
A)MgO + HCl → MgCl2 + H2O
B)Al + Fe2O3 → Al2O3 + Fe
C)KClO3 → KCl + O2
D)CH4 + O2 → CO2 + H2O
A)MgO + HCl → MgCl2 + H2O
B)Al + Fe2O3 → Al2O3 + Fe
C)KClO3 → KCl + O2
D)CH4 + O2 → CO2 + H2O

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following equations is not a single replacement reaction?
A)H2SO4 + Mg(OH)2 → H2O + MgSO4
B)Zn + HCl → H2 + ZnCl2
C)Al + Fe2O3 → Al2O3 + Fe
D)Na + H2O → NaOH + H2
A)H2SO4 + Mg(OH)2 → H2O + MgSO4
B)Zn + HCl → H2 + ZnCl2
C)Al + Fe2O3 → Al2O3 + Fe
D)Na + H2O → NaOH + H2

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following equations is not a combustion reaction?
A)C2H6O + O2 → H2O + CO2
B)CH4 + O2 → CO + H2O
C)KClO3 → KCl +O2
D)Fe + O2 → FeO
A)C2H6O + O2 → H2O + CO2
B)CH4 + O2 → CO + H2O
C)KClO3 → KCl +O2
D)Fe + O2 → FeO

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
15
Which process releases energy?
A)bond formation
B)bond breaking
C)both A and B
D)none of the above
A)bond formation
B)bond breaking
C)both A and B
D)none of the above

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
16
The spectator ions in the precipitation equation AgNO3 + NaCl → AgCl + NaNO3 are ________.
A)Ag+ and NO3-
B)Na+ and NO3-
C)Ag+ and Cl-
D)Na+ and Cl-
A)Ag+ and NO3-
B)Na+ and NO3-
C)Ag+ and Cl-
D)Na+ and Cl-

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following equations is not a decomposition reaction?
A)CaSO4 ∙ 2H2O → CaSO4 + 2 H2O
B)NaClO3 → NaCl + O2
C)H2O2 → H2O + O2
D)K + H2O → KOH + H2
A)CaSO4 ∙ 2H2O → CaSO4 + 2 H2O
B)NaClO3 → NaCl + O2
C)H2O2 → H2O + O2
D)K + H2O → KOH + H2

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is an example of a synthesis reaction?
A)HCl + NaOH → NaCl + H2O
B)Mg + HCl → H2 + MgCl2
C)2 Na + Cl2 → 2NaCl
D)KClO3 → KCl + O2
A)HCl + NaOH → NaCl + H2O
B)Mg + HCl → H2 + MgCl2
C)2 Na + Cl2 → 2NaCl
D)KClO3 → KCl + O2

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
19
Which ions are not spectator ions in the precipitation equation AgNO3 + NaCl → AgCl + NaNO3?
A)Ag+ and NO3-
B)Na+ and NO3-
C)Ag+ and Cl-
D)Na+ and Cl-
A)Ag+ and NO3-
B)Na+ and NO3-
C)Ag+ and Cl-
D)Na+ and Cl-

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements is correct about a superconductor?
A)It possesses the extraordinary property, when cooled, to have a magnet float as if defying gravity.
B)It conducts electricity with zero resistance.
C)It prevents magnetic field lines from penetrating it.
D)All of the above statements are correct about a superconductor.
A)It possesses the extraordinary property, when cooled, to have a magnet float as if defying gravity.
B)It conducts electricity with zero resistance.
C)It prevents magnetic field lines from penetrating it.
D)All of the above statements are correct about a superconductor.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following equations does not have 1:2:1:2 as the relative ratio of coefficients when balanced?
A)H2SO4 + KOH → K2SO4 + H2O
B)Ca(OH)2 + HCl → CaCl2 + H2O
C)CH4 + O2 → CO2 + H2O
D)K + H2O → H2 + KOH
A)H2SO4 + KOH → K2SO4 + H2O
B)Ca(OH)2 + HCl → CaCl2 + H2O
C)CH4 + O2 → CO2 + H2O
D)K + H2O → H2 + KOH

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
22
When aqueous solutions of calcium hydroxide and hydrobromic acid are mixed, the spectator ions are ________.
A)calcium and bromide
B)hydrogen and hydroxide
C)calcium and hydroxide
D)hydrogen and bromide
A)calcium and bromide
B)hydrogen and hydroxide
C)calcium and hydroxide
D)hydrogen and bromide

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
23
After balancing the equation Fe2O3 + Al → Al2O3 + Fe the correct coefficients are respectively ________.
A)1: 2: 1: 2
B)2: 3: 2: 3
C)1: 2: 2: 1
D)2: 3: 3: 4
A)1: 2: 1: 2
B)2: 3: 2: 3
C)1: 2: 2: 1
D)2: 3: 3: 4

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
24
The equation C8H18 + O2 → CO2 + H2O is an example of a ________ reaction.
A)synthesis
B)decomposition
C)double displacement
D)combustion
A)synthesis
B)decomposition
C)double displacement
D)combustion

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following balanced reactions does not have at least one 6 as a coefficient?
A)H2SO4 + Al(OH)3 → Al2(SO4)3 + H2O
B)H3PO4 + Ba(OH)2 → Ba3(PO4)2 + H2O
C)C6H12O6 + O2 → CO2 + H2O
D)C2H2 + O2 → CO2 + H2O
A)H2SO4 + Al(OH)3 → Al2(SO4)3 + H2O
B)H3PO4 + Ba(OH)2 → Ba3(PO4)2 + H2O
C)C6H12O6 + O2 → CO2 + H2O
D)C2H2 + O2 → CO2 + H2O

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
26
If the following ions Ca2+, Cl-, Na+ and CO32- are placed in a test tube, the precipitate formed is ________.
A)CaCO3
B)NaCl
C)CaCl2
D)no precipitate will be formed
A)CaCO3
B)NaCl
C)CaCl2
D)no precipitate will be formed

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
27
The precipitate formed when barium nitrate and potassium chromate are placed in a test tube is ________.
A)barium nitrate
B)barium chromate
C)potassium nitrate
D)potassium chromate
A)barium nitrate
B)barium chromate
C)potassium nitrate
D)potassium chromate

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
28
What is the sum of all coefficients after the following equation is balanced C8H18 + O2 → CO2 + H2O?
A)4
B)18
C)32
D)61
A)4
B)18
C)32
D)61

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
29
What is the coefficient for iron after the following reaction is balanced Fe + O2 → Fe2O3?
A)1
B)3
C)4
D)6
A)1
B)3
C)4
D)6

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following equations has 1:1:1:1 as the relative ratio of coefficients when balanced?
A)H2SO4 + KOH → K2SO4 + H2O
B)Zn + HCl → H2 + ZnCl2
C)CH4 + O2 → CO2 + H2O
D)NaOH + HCl → NaCl + H2O
A)H2SO4 + KOH → K2SO4 + H2O
B)Zn + HCl → H2 + ZnCl2
C)CH4 + O2 → CO2 + H2O
D)NaOH + HCl → NaCl + H2O

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
31
The precipitate formed when sodium chloride and lead (II)nitrate are placed in a test tube is ________.
A)lead (II)chloride
B)sodium nitrate
C)lead (II)hydroxide
D)No precipitate will be formed.
A)lead (II)chloride
B)sodium nitrate
C)lead (II)hydroxide
D)No precipitate will be formed.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following salts is water insoluble?
A)K2SO4
B)AgNO3
C)CaCO3
D)Na3PO4
A)K2SO4
B)AgNO3
C)CaCO3
D)Na3PO4

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
33
The net ionic equation for the reaction AgNO3 + NaCl → AgCl + NaNO3 is ________.
A)Ag+ (aq)+ NO3- (aq)→ AgNO3
B)Ag+ (aq)+ Cl- (aq)→ AgCl (s)
C)Na+ (aq)+ NO3- (aq)→ NaNO3
D)NaCl + AgNO3 → AgCl (s)
A)Ag+ (aq)+ NO3- (aq)→ AgNO3
B)Ag+ (aq)+ Cl- (aq)→ AgCl (s)
C)Na+ (aq)+ NO3- (aq)→ NaNO3
D)NaCl + AgNO3 → AgCl (s)

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following equations has 1:2:1:1 as the relative ratio of coefficients when balanced?
A)H2SO4 + KOH → K2SO4 + H2O
B)Zn + HCl → H2 + ZnCl2
C)CH4 + O2 → CO2 + H2O
D)NaOH + HCl → NaCl + H2O
A)H2SO4 + KOH → K2SO4 + H2O
B)Zn + HCl → H2 + ZnCl2
C)CH4 + O2 → CO2 + H2O
D)NaOH + HCl → NaCl + H2O

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following has 6 as one of the coefficients when the equation is balanced?
A)H2SO4 + Al(OH)3 → Al2(SO4)3 + H2O
B)C2H6O +O2 → CO2 + H2O
C)Mg + HCl → H2 + MgCl2
D)Al(OH)3 + HCl → AlCl3 + H2O
A)H2SO4 + Al(OH)3 → Al2(SO4)3 + H2O
B)C2H6O +O2 → CO2 + H2O
C)Mg + HCl → H2 + MgCl2
D)Al(OH)3 + HCl → AlCl3 + H2O

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
36
The net ionic equation for the reaction H2SO4 + KOH → K2SO4 + H2O is ________.
A)K+ (aq)+ SO42- (aq)→ K2SO4
B)H+ (aq)+ OH- (aq)→ H2O
C)K+ (aq)+ OH-(aq)→ KOH
D)H+ (aq)+ SO42- (aq)→ H2SO4
A)K+ (aq)+ SO42- (aq)→ K2SO4
B)H+ (aq)+ OH- (aq)→ H2O
C)K+ (aq)+ OH-(aq)→ KOH
D)H+ (aq)+ SO42- (aq)→ H2SO4

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
37
When aqueous solutions of sodium nitrate and potassium chloride are mixed, the spectator ions are ________.
A)potassium and nitrate
B)sodium and chloride
C)both A and B
D)no spectator ions are present
A)potassium and nitrate
B)sodium and chloride
C)both A and B
D)no spectator ions are present

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is the least soluble in water?
A)sodium sulfate
B)lead (II)nitrate
C)potassium chloride
D)calcium sulfate
A)sodium sulfate
B)lead (II)nitrate
C)potassium chloride
D)calcium sulfate

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
39
If the following ions Ba2+, Cl-, K+ and SO42- are placed in a test tube, the precipitate formed is ________.
A)BaSO4
B)KCl
C)BaCl2
D)K2SO4
A)BaSO4
B)KCl
C)BaCl2
D)K2SO4

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
40
When aqueous solutions of sodium phosphate and calcium nitrite are mixed, the spectator ions are ________.
A)calcium and nitrite
B)sodium and phosphate
C)calcium and phosphate
D)sodium and nitrite
A)calcium and nitrite
B)sodium and phosphate
C)calcium and phosphate
D)sodium and nitrite

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
41
Which sulfate is water soluble?
A)calcium sulfate
B)barium sulfate
C)lithium sulfate
D)lead (II)sulfate
A)calcium sulfate
B)barium sulfate
C)lithium sulfate
D)lead (II)sulfate

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
42
The number of moles of sodium hydroxide needed to neutralize one mole of nitric acid is ________.
A)1
B)2
C)3
D)0.5
A)1
B)2
C)3
D)0.5

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
43
Which sulfide is water insoluble?
A)lead (II)sulfide
B)potassium sulfide
C)ammonium sulfide
D)sodium sulfide
A)lead (II)sulfide
B)potassium sulfide
C)ammonium sulfide
D)sodium sulfide

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
44
Which sulfate is water insoluble?
A)ammonium sulfate
B)strontium sulfate
C)sodium sulfate
D)potassium sulfate
A)ammonium sulfate
B)strontium sulfate
C)sodium sulfate
D)potassium sulfate

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
45
In a decomposition reaction one compound breaks down into two or more simpler compounds or elements.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following salts is water soluble?
A)AgCl
B)BaCO3
C)Al2(SO4)3
D)NaNO3
A)AgCl
B)BaCO3
C)Al2(SO4)3
D)NaNO3

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
47
In a decomposition reaction one compound breaks down into two or more simpler compounds or elements.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
48
Which hydroxide is water insoluble?
A)sodium hydroxide
B)barium hydroxide
C)aluminum hydroxide
D)calcium hydroxide
A)sodium hydroxide
B)barium hydroxide
C)aluminum hydroxide
D)calcium hydroxide

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
49
A combustion reaction always involves carbon dioxide formation.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
50
Bond formation releases energy.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
51
It takes ________ moles of calcium hydroxide to neutralize ________ moles of sulfuric acid.
A)1; 1
B)1; 2
C)2; 1
D)1; 3
A)1; 1
B)1; 2
C)2; 1
D)1; 3

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
52
In a combustion reaction, oxygen is typically one of the reactants.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
53
Bond breaking releases energy.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
54
All nitrates are water soluble

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
55
In a combination reaction two or more compounds yield two or more compounds.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
56
Which phosphate is water soluble?
A)ammonium phosphate
B)cobalt (II)phosphate
C)aluminum phosphate
D)barium phosphate
A)ammonium phosphate
B)cobalt (II)phosphate
C)aluminum phosphate
D)barium phosphate

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
57
Which chloride is water soluble?
A)lead (II)chloride
B)calcium chloride
C)mercury (I)chloride
D)silver (I)chloride
A)lead (II)chloride
B)calcium chloride
C)mercury (I)chloride
D)silver (I)chloride

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
58
It takes ________ moles of potassium hydroxide to neutralize ________ moles of sulfuric acid.
A)1; 1
B)1; 2
C)2; 1
D)3; 1
A)1; 1
B)1; 2
C)2; 1
D)3; 1

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
59
It takes ________ moles of sulfuric acid to neutralize ________ moles of aluminum hydroxide.
A)2; 3
B)3; 2
C)1; 2
D)1; 1
A)2; 3
B)3; 2
C)1; 2
D)1; 1

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
60
One needs to balance a chemical reaction before identifying the type of reaction.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
61
Match between columns

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
62
An alternative name for lye is potassium hydroxide.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
63
Match between columns
Premises:
potassium nitrate
potassium nitrate
lead (II) sulfate
lead (II) sulfate
Responses:
water soluble
water insoluble
water soluble
water insoluble
water soluble
water insoluble
water soluble
water insoluble
water soluble
water insoluble
water soluble
water insoluble
water soluble
water insoluble
water soluble
water insoluble
water soluble
water insoluble

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
64
The coefficients in the balanced equation KClO3 → KCl + O2 are 2: 2: 3.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
65
Match between columns

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
66
Match between columns

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
67
Match between columns
Premises:
H₂ + O₂ → H₂O
H₂ + O₂ → H₂O
H₂ + O₂ → H₂O
H₂ + O₂ → H₂O
H₂ + O₂ → H₂O
H₂ + O₂ → H₂O
NaClO₃ → NaCl + O₂
NaClO₃ → NaCl + O₂
NaClO₃ → NaCl + O₂
NaClO₃ → NaCl + O₂
NaClO₃ → NaCl + O₂
NaClO₃ → NaCl + O₂
CH₄ + O₂ → CO₂ + H₂O
CH₄ + O₂ → CO₂ + H₂O
CH₄ + O₂ → CO₂ + H₂O
CH₄ + O₂ → CO₂ + H₂O
CH₄ + O₂ → CO₂ + H₂O
CH₄ + O₂ → CO₂ + H₂O
Mg + HCl → H₂ + MgCl₂
Mg + HCl → H₂ + MgCl₂
Mg + HCl → H₂ + MgCl₂
Mg + HCl → H₂ + MgCl₂
Mg + HCl → H₂ + MgCl₂
Mg + HCl → H₂ + MgCl₂
Na₂SO₄ + PbCl₂ → PbSO₄
Na₂SO₄ + PbCl₂ → PbSO₄
Na₂SO₄ + PbCl₂ → PbSO₄
Na₂SO₄ + PbCl₂ → PbSO₄
Na₂SO₄ + PbCl₂ → PbSO₄
Na₂SO₄ + PbCl₂ → PbSO₄
H₂SO₄ + NaOH → Na₂SO₄ + H₂O
H₂SO₄ + NaOH → Na₂SO₄ + H₂O
H₂SO₄ + NaOH → Na₂SO₄ + H₂O
H₂SO₄ + NaOH → Na₂SO₄ + H₂O
H₂SO₄ + NaOH → Na₂SO₄ + H₂O
H₂SO₄ + NaOH → Na₂SO₄ + H₂O
Responses:
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement
Single replacement
Combustion
Decomposition
Combination
Precipitation
Double replacement

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
68
Silver (I)chloride is water insoluble while silver (I)acetate is water soluble.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
69
It takes two moles of sulfuric acid to neutralize one mole of sodium hydroxide.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
70
An alternative name for hydrochloric acid is muriatic acid.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
71
All hydroxides are water insoluble

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
71
Match every equation in the questions with its type that appears in the list below. 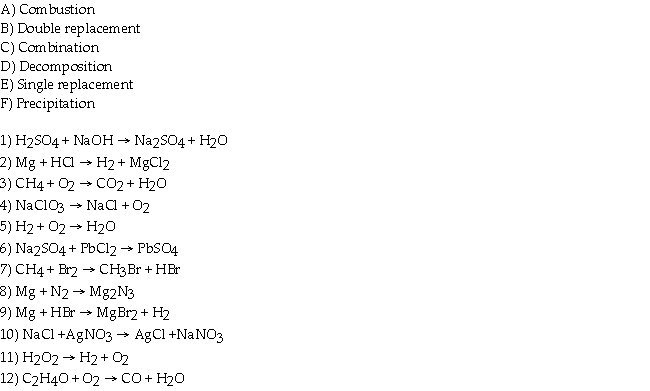
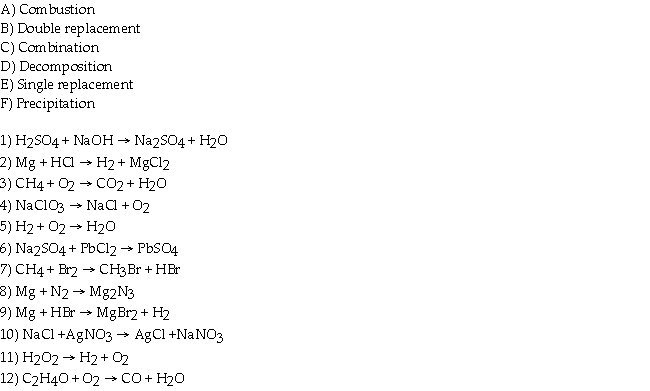

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
72
Ammonium phosphate is water soluble but ammonium carbonate is water insoluble.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
73
Predict whether each of the compounds listed in the questions is water soluble or insoluble. 


Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
73
The coefficients in the balanced equation C8H6 + O2 → CO2 + H2O are 1: 19: 8: 3.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
74
Match each of the acid-base pairs in the questions with the relative neutralization ratio that appears in the list below. 


Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
74
Only the halides of lead (II)and silver (I)are water insoluble.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
75
Match each of the acids that appears in the questions with a use or alternative name that appears in the list below. 


Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
75
When the following ions barium, chloride, sodium and sulfate are placed together in a test tube, barium sulfate precipitates out.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck



