Deck 10: Chemical Bonding Ii: Molecular Shapes,valence Bond Theory,
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/144
Play
Full screen (f)
Deck 10: Chemical Bonding Ii: Molecular Shapes,valence Bond Theory,
1
If an oil tanker leaks oil in the ocean,where does the oil go?
A) The oil floats on the seawater; water is polar and oil is nonpolar.
B) The oil mixes with the water.
C) The oil sinks below the seawater; water is nonpolar and oil is polar.
D) The oil floats on the seawater; water is nonpolar and oil is polar.
E) The oil sinks below the seawater; water is polar and oil is nonpolar.
A) The oil floats on the seawater; water is polar and oil is nonpolar.
B) The oil mixes with the water.
C) The oil sinks below the seawater; water is nonpolar and oil is polar.
D) The oil floats on the seawater; water is nonpolar and oil is polar.
E) The oil sinks below the seawater; water is polar and oil is nonpolar.
The oil floats on the seawater; water is polar and oil is nonpolar.
2
Identify the number of electron groups around a molecule with sp hybridization.
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5
2
3
Place the following in order of decreasing dipole moment. I.cis-CHCl=CHCl II.trans-CHCl=CHCl III.cis-CHF=CHF
A) III > I > II
B) II > I > III
C) I > III > II
D) II > III > I
E) I = III > II
A) III > I > II
B) II > I > III
C) I > III > II
D) II > III > I
E) I = III > II
III > I > II
4
Give the molecular geometry and number of electron groups for SF4.
A) square planar, 6 electron groups
B) square pyramidal, 6 electron groups
C) T-shaped, 5 electron groups
D) octahedral, 6 electron groups
E) seesaw, 5 electron groups
A) square planar, 6 electron groups
B) square pyramidal, 6 electron groups
C) T-shaped, 5 electron groups
D) octahedral, 6 electron groups
E) seesaw, 5 electron groups

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
5
Soap works with water because
A) the polar head and the nonpolar tail attracts water.
B) the polar head attracts the grease and the nonpolar tail attracts water.
C) the polar head attracts water and the nonpolar tail attracts the grease.
D) the polar head and the nonpolar tail attracts the grease.
A) the polar head and the nonpolar tail attracts water.
B) the polar head attracts the grease and the nonpolar tail attracts water.
C) the polar head attracts water and the nonpolar tail attracts the grease.
D) the polar head and the nonpolar tail attracts the grease.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
6
Place the following in order of increasing dipole moment. I.BCl3 II.BIF2 III.BClF2
A) I < II = III
B) II < III < I
C) I < II < III
D) II < I < III
E) I < III < II
A) I < II = III
B) II < III < I
C) I < II < III
D) II < I < III
E) I < III < II

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
7
Place the following in order of decreasing X-A-X bond angle,where A represents the central atom and X represents the outer atoms in each molecule. N2O NCl3 NO2⁻
A) NCl3 > NO2⁻ > N2O
B) NO2⁻ > N2O > NCl3
C) N2O > NO2⁻ > NCl3
D) NCl3 > N2O > NO2⁻
E) N2O > NCl3 > NO2⁻
A) NCl3 > NO2⁻ > N2O
B) NO2⁻ > N2O > NCl3
C) N2O > NO2⁻ > NCl3
D) NCl3 > N2O > NO2⁻
E) N2O > NCl3 > NO2⁻

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
8
A molecule with a T-shaped molecular geometry has a bond angle of
A) <120° for equatorial bonds and <90° for axial bonds.
B) 180°.
C) <90°.
D) 120° for equatorial bonds and 90° for axial bonds.
E) 120°.
A) <120° for equatorial bonds and <90° for axial bonds.
B) 180°.
C) <90°.
D) 120° for equatorial bonds and 90° for axial bonds.
E) 120°.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
9
A molecule with a trigonal bipyramidal molecular geometry has a bond angle of
A) <120° for equatorial bonds and <90° for axial bonds.
B) 180°.
C) <90°.
D) 120° for equatorial bonds and 90° for axial bonds.
E) 120°.
A) <120° for equatorial bonds and <90° for axial bonds.
B) 180°.
C) <90°.
D) 120° for equatorial bonds and 90° for axial bonds.
E) 120°.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
10
Give the molecular geometry and number of electron groups for BrF5.
A) square planar, 6 electron groups
B) square pyramidal, 6 electron groups
C) T-shaped, 5 electron groups
D) octahedral, 6 electron groups
E) seesaw, 5 electron groups
A) square planar, 6 electron groups
B) square pyramidal, 6 electron groups
C) T-shaped, 5 electron groups
D) octahedral, 6 electron groups
E) seesaw, 5 electron groups

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
11
Place the following in order of decreasing X-A-X bond angle,where A represents the central atom and X represents the outer atoms in each molecule. CS2 CF4 SCl2
A) CS2 = SCl2 > CF4
B) SCl2 > CF4 > CS2
C) CF4 > CS2 > SCl2
D) CS2 > CF4 > SCl2
E) CF4 > CS2 > SCl2
A) CS2 = SCl2 > CF4
B) SCl2 > CF4 > CS2
C) CF4 > CS2 > SCl2
D) CS2 > CF4 > SCl2
E) CF4 > CS2 > SCl2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
12
Place the following in order of increasing X-Se-X bond angle,where X represents the outer atoms in each molecule. SeO2 SeCl6 SeF2
A) SeCl6 < SeF2 < SeO2
B) SeF2 < SeO2 < SeCl6
C) SeF2 < SeCl6 < SeO2
D) SeO2 < SeF2 < SeCl6
E) SeCl6 < SeO2 < SeF2
A) SeCl6 < SeF2 < SeO2
B) SeF2 < SeO2 < SeCl6
C) SeF2 < SeCl6 < SeO2
D) SeO2 < SeF2 < SeCl6
E) SeCl6 < SeO2 < SeF2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
13
How many of the following molecules are polar? BrCl3 CS2 SiF4 SO3
A) 1
B) 2
C) 3
D) 4
E) 0
A) 1
B) 2
C) 3
D) 4
E) 0

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
14
A molecule with a seesaw molecular geometry has a bond angle of
A) <120° for equatorial bonds and <90° for axial bonds.
B) 180°.
C) <90°.
D) 120° for equatorial bonds and 90° for axial bonds.
E) 120°.
A) <120° for equatorial bonds and <90° for axial bonds.
B) 180°.
C) <90°.
D) 120° for equatorial bonds and 90° for axial bonds.
E) 120°.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
15
Consider the molecule below.Determine the molecular geometry at each of the 3 labeled atoms. 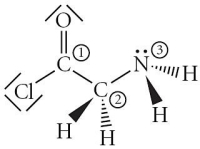
A) 1 = trigonal planar, 2 = tetrahedral, 3 = trigonal pyramidal
B) 1 = tetrahedral, 2 = tetrahedral, 3 = tetrahedral
C) 1 = trigonal planar, 2 = tetrahedral, 3 = tetrahedral
D) 1 = tetrahedral, 2 = tetrahedral, 3 = trigonal planar
E) 1 = trigonal planar, 2 = trigonal pyramidal, 3 = trigonal pyramidal

A) 1 = trigonal planar, 2 = tetrahedral, 3 = trigonal pyramidal
B) 1 = tetrahedral, 2 = tetrahedral, 3 = tetrahedral
C) 1 = trigonal planar, 2 = tetrahedral, 3 = tetrahedral
D) 1 = tetrahedral, 2 = tetrahedral, 3 = trigonal planar
E) 1 = trigonal planar, 2 = trigonal pyramidal, 3 = trigonal pyramidal

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
16
Place the following in order of increasing F-A-F bond angle,where A represents the central atom in each molecule. PF3 OF2 PF4⁺
A) PF3 < OF2 < PF4⁺
B) OF2 < PF3 < PF4⁺
C) OF2 < PF4⁺ < PF3
D) PF4⁺ < OF2 < PF3
E) PF4⁺ < PF3 < OF2
A) PF3 < OF2 < PF4⁺
B) OF2 < PF3 < PF4⁺
C) OF2 < PF4⁺ < PF3
D) PF4⁺ < OF2 < PF3
E) PF4⁺ < PF3 < OF2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
17
Consider the molecule below.Determine the molecular geometry at each of the 2 labeled carbons. 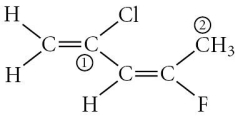
A) C1 = tetrahedral, C2 = linear
B) C1 = trigonal planar, C2= bent
C) C1 = bent, C2 = trigonal planar
D) C1 = trigonal planar, C2 = tetrahedral
E) C1 = trigonal pyramidal, C2 = see-saw

A) C1 = tetrahedral, C2 = linear
B) C1 = trigonal planar, C2= bent
C) C1 = bent, C2 = trigonal planar
D) C1 = trigonal planar, C2 = tetrahedral
E) C1 = trigonal pyramidal, C2 = see-saw

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
18
Give the molecular geometry and number of electron groups for BrF3.
A) square planar, 6 electron groups
B) square pyramidal, 6 electron groups
C) T-shaped, 5 electron groups
D) octahedral, 6 electron groups
E) seesaw, 5 electron groups
A) square planar, 6 electron groups
B) square pyramidal, 6 electron groups
C) T-shaped, 5 electron groups
D) octahedral, 6 electron groups
E) seesaw, 5 electron groups

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
19
Place the following in order of increasing X-A-X bond angle,where A represents the central atom and X represents the outer atoms in each molecule. HCN H2O H3O⁺
A) H3O⁺ < HCN < H2O
B) H3O⁺ < H2O < HCN
C) HCN < H3O⁺ < H2O
D) H2O < HCN < H3O⁺
E) H2O < H3O⁺ < HCN
A) H3O⁺ < HCN < H2O
B) H3O⁺ < H2O < HCN
C) HCN < H3O⁺ < H2O
D) H2O < HCN < H3O⁺
E) H2O < H3O⁺ < HCN

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
20
How many of the following molecules are polar? PCl5 COS XeO3 SeBr2
A) 2
B) 0
C) 1
D) 3
E) 4
A) 2
B) 0
C) 1
D) 3
E) 4

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
21
How many of the following molecules have sp2 hybridization on the central atom? HCN SO2 OCl2 XeCl2
A) 4
B) 3
C) 2
D) 1
E) 0
A) 4
B) 3
C) 2
D) 1
E) 0

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
22
Give the hybridization for the S in SF6.
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
23
A molecule,that is sp3d hybridized and has a molecular geometry of seesaw,has ________ bonding groups and ________ lone pairs around its central atom.
A) 5, 1
B) 4, 2
C) 4, 1
D) 3, 2
E) 2, 3
A) 5, 1
B) 4, 2
C) 4, 1
D) 3, 2
E) 2, 3

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
24
A molecule,that is sp3d hybridized and has a molecular geometry of linear,has ________ bonding groups and ________ lone pairs around its central atom.
A) 5, 1
B) 4, 2
C) 4, 1
D) 3, 2
E) 2, 3
A) 5, 1
B) 4, 2
C) 4, 1
D) 3, 2
E) 2, 3

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
25
A molecule,that is sp3d2 hybridized and has a molecular geometry of square planar,has ________ bonding groups and ________ lone pairs around its central atom.
A) 5, 1
B) 4, 2
C) 4, 1
D) 3, 2
E) 2, 3
A) 5, 1
B) 4, 2
C) 4, 1
D) 3, 2
E) 2, 3

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
26
Give the hybridization for the O in H3O+.
A) sp
B) sp3
C) sp2
D) sp3d
E) sp3d2
A) sp
B) sp3
C) sp2
D) sp3d
E) sp3d2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
27
A molecule,that is sp3d2 hybridized and has a molecular geometry of square pyramidal,has ________ bonding groups and ________ lone pairs around its central atom.
A) 5, 1
B) 4, 2
C) 4, 1
D) 3, 2
E) 2, 3
A) 5, 1
B) 4, 2
C) 4, 1
D) 3, 2
E) 2, 3

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
28
Identify the number of electron groups around a molecule with sp2 hybridization.
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
29
Identify the number of electron groups around a molecule with sp3d hybridization.
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following statements is TRUE?
A) The total number of molecular orbitals formed doesn't always equal the number of atomic orbitals in the set.
B) A bond order of 0 represents a stable chemical bond.
C) When two atomic orbitals come together to form two molecular orbitals, one molecular orbital will be lower in energy than the two separate atomic orbitals and one molecular orbital will be higher in energy than the separate atomic orbitals.
D) Electrons placed in antibonding orbitals stabilize the ion/molecule.
E) All of the above are true.
A) The total number of molecular orbitals formed doesn't always equal the number of atomic orbitals in the set.
B) A bond order of 0 represents a stable chemical bond.
C) When two atomic orbitals come together to form two molecular orbitals, one molecular orbital will be lower in energy than the two separate atomic orbitals and one molecular orbital will be higher in energy than the separate atomic orbitals.
D) Electrons placed in antibonding orbitals stabilize the ion/molecule.
E) All of the above are true.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
31
Identify the number of electron groups around a molecule with sp3d2 hybridization.
A) 6
B) 2
C) 3
D) 4
E) 5
A) 6
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
32
Give the hybridization for the Br in BrCl3.
A) sp3d2
B) sp3d
C) sp3
D) sp2
E) sp
A) sp3d2
B) sp3d
C) sp3
D) sp2
E) sp

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
33
Give the hybridization for the Br in BrO4⁻.
A) sp
B) sp3d2
C) sp3d
D) sp3
E) sp2
A) sp
B) sp3d2
C) sp3d
D) sp3
E) sp2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
34
Give the hybridization for the Br in BrF5.
A) sp3d2
B) sp3d
C) sp3
D) sp2
E) sp
A) sp3d2
B) sp3d
C) sp3
D) sp2
E) sp

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
35
Give the hybridization for the O in OF2.
A) sp
B) sp3
C) sp2
D) sp3d
E) sp3d2
A) sp
B) sp3
C) sp2
D) sp3d
E) sp3d2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
36
Give the hybridization for the S in SO3.
A) sp
B) sp3
C) sp2
D) sp3d
E) sp3d2
A) sp
B) sp3
C) sp2
D) sp3d
E) sp3d2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
37
Give the hybridization for the C in HCCH.
A) sp3d2
B) sp3d
C) sp3
D) sp2
E) sp
A) sp3d2
B) sp3d
C) sp3
D) sp2
E) sp

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
38
A molecule,that is sp3d hybridized and has a molecular geometry of T-shaped,has ________ bonding groups and ________ lone pairs around its central atom.
A) 5, 1
B) 4, 2
C) 4, 1
D) 3, 2
E) 2, 3
A) 5, 1
B) 4, 2
C) 4, 1
D) 3, 2
E) 2, 3

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
39
Identify the number of electron groups around a molecule with sp3 hybridization.
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
40
Give the hybridization for the C in HCN.
A) sp3d2
B) sp3d
C) sp3
D) sp2
E) sp
A) sp3d2
B) sp3d
C) sp3
D) sp2
E) sp

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
41
Draw the molecular orbital diagram shown to determine which of the following is most stable.
A) C22⁺
B) N22⁺
C) B2
D) C22⁻
E) B22⁺
A) C22⁺
B) N22⁺
C) B2
D) C22⁻
E) B22⁺

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
42
Give the number of sigma bonds and pi bonds for benzene,C6H6.
A) 3 sigma bonds, 6 pi bonds
B) 6 sigma bonds, 6 pi bonds
C) 6 sigma bonds, 3 pi bonds
D) 12 sigma bonds, 3 pi bonds
E) 15 sigma bonds, 3 pi bonds
A) 3 sigma bonds, 6 pi bonds
B) 6 sigma bonds, 6 pi bonds
C) 6 sigma bonds, 3 pi bonds
D) 12 sigma bonds, 3 pi bonds
E) 15 sigma bonds, 3 pi bonds

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
43
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
A) O22⁻
B) Ne22⁺
C) O22⁺
D) F22⁺
E) None of the above is paramagnetic.
A) O22⁻
B) Ne22⁺
C) O22⁺
D) F22⁺
E) None of the above is paramagnetic.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
44
Determine the electron geometry (eg)and molecular geometry (mg)of CF3+1.
A) eg = tetrahedral, mg = tetrahedral
B) eg = trigonal pyramidal, mg = trigonal pyramidal
C) eg = bent, mg = bent
D) eg = trigonal planar, mg = trigonal planar
E) eg = tetrahedral, mg = trigonal planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = trigonal pyramidal, mg = trigonal pyramidal
C) eg = bent, mg = bent
D) eg = trigonal planar, mg = trigonal planar
E) eg = tetrahedral, mg = trigonal planar

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
45
Give the approximate bond angle for a molecule with a trigonal planar shape.
A) 109.5°
B) 180°
C) 120°
D) 107°
E) 45°
A) 109.5°
B) 180°
C) 120°
D) 107°
E) 45°

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
46
Use the molecular orbital diagram shown to determine which of the following is most stable. 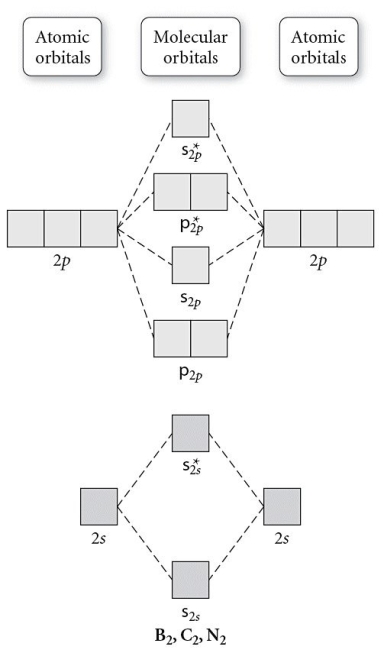
A) C22⁺
B) N22⁺
C) B2
D) C22⁻
E) B22⁺

A) C22⁺
B) N22⁺
C) B2
D) C22⁻
E) B22⁺

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
47
Give the approximate bond angle for a molecule with a tetrahedral shape.
A) 109.5°
B) 200°
C) 120°
D) 107°
E) 45°
A) 109.5°
B) 200°
C) 120°
D) 107°
E) 45°

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
48
Determine the electron geometry (eg)and molecular geometry (mg)of SiBr4.
A) eg = tetrahedral, mg = trigonal pyramidal
B) eg = octahedral, mg = square planar
C) eg = trigonal bipyramidal, mg = trigonal bipyramidal
D) eg = tetrahedral, mg = bent
E) eg = tetrahedral, mg = tetrahedral
A) eg = tetrahedral, mg = trigonal pyramidal
B) eg = octahedral, mg = square planar
C) eg = trigonal bipyramidal, mg = trigonal bipyramidal
D) eg = tetrahedral, mg = bent
E) eg = tetrahedral, mg = tetrahedral

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
49
Determine the electron geometry (eg)and molecular geometry (mg)of BH3.
A) eg = trigonal planar, mg = trigonal planar
B) eg = tetrahedral, mg = trigonal planar
C) eg = tetrahedral, mg = trigonal pyramidal
D) eg = trigonal planar, mg = tetrahedral
E) eg = trigonal bipyramidal, mg = trigonal bipyramidal
A) eg = trigonal planar, mg = trigonal planar
B) eg = tetrahedral, mg = trigonal planar
C) eg = tetrahedral, mg = trigonal pyramidal
D) eg = trigonal planar, mg = tetrahedral
E) eg = trigonal bipyramidal, mg = trigonal bipyramidal

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
50
Determine the electron geometry (eg)and molecular geometry (mg)of PF5.
A) eg = trigonal bipyramidal, mg = trigonal bipyramidal
B) eg = octahedral, mg = square pyramidal
C) eg = trigonal bipyramidal, mg = tetrahedral
D) eg = tetrahedral, mg = octahedral
E) eg = trigonal planar, mg = octahedral
A) eg = trigonal bipyramidal, mg = trigonal bipyramidal
B) eg = octahedral, mg = square pyramidal
C) eg = trigonal bipyramidal, mg = tetrahedral
D) eg = tetrahedral, mg = octahedral
E) eg = trigonal planar, mg = octahedral

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
51
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
A) B22⁺
B) B22⁻
C) N22⁺
D) C22⁻
E) B2
A) B22⁺
B) B22⁻
C) N22⁺
D) C22⁻
E) B2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
52
Determine the electron geometry (eg)and molecular geometry (mg)of CO32⁻.
A) eg = tetrahedral, mg = planar
B) eg = tetrahedral, mg = trigonal bipyramidal
C) eg = trigonal planar, mg = tetrahedral
D) eg = trigonal planar, mg = trigonal planar
E) eg = octahedral, mg = trigonal pyramidal
A) eg = tetrahedral, mg = planar
B) eg = tetrahedral, mg = trigonal bipyramidal
C) eg = trigonal planar, mg = tetrahedral
D) eg = trigonal planar, mg = trigonal planar
E) eg = octahedral, mg = trigonal pyramidal

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
53
Identify the hybridization for the carbons in benzene,C6H6.
A) sp3d2
B) sp3d
C) sp3
D) sp
E) sp2
A) sp3d2
B) sp3d
C) sp3
D) sp
E) sp2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
54
Use the molecular orbital diagram shown to determine which of the following is paramagnetic. 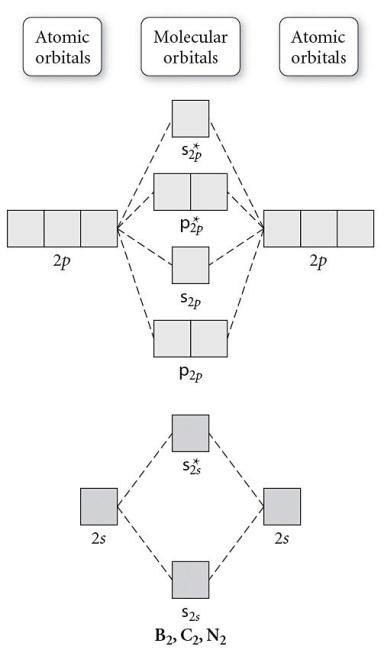
A) B22⁺
B) B22⁻
C) N22⁺
D) C22⁻
E) B2

A) B22⁺
B) B22⁻
C) N22⁺
D) C22⁻
E) B2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
55
Use the molecular orbital diagram shown to determine which of the following is paramagnetic. 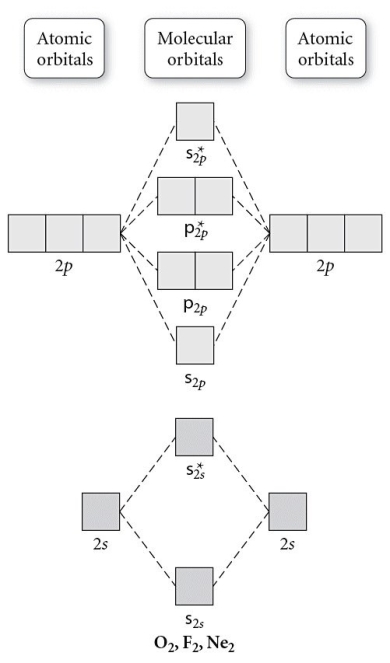
A) O22⁻
B) Ne22⁺
C) O22⁺
D) F22⁺
E) None of the above is paramagnetic.

A) O22⁻
B) Ne22⁺
C) O22⁺
D) F22⁺
E) None of the above is paramagnetic.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
56
What geometric arrangement of charge clouds is expected for an atom that has three charge clouds?
A) trigonal bipyramidal
B) linear
C) trigonal
D) tetrahedral
A) trigonal bipyramidal
B) linear
C) trigonal
D) tetrahedral

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
57
Draw the molecular orbital diagram shown to determine which of the following is most stable.
A) F2
B) F22⁺
C) Ne22⁺
D) O22⁺
E) F22⁻
A) F2
B) F22⁺
C) Ne22⁺
D) O22⁺
E) F22⁻

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
58
Give the approximate bond angle for a molecule with a linear shape.
A) 109.5°
B) 180°
C) 120°
D) 105°
E) 60°
A) 109.5°
B) 180°
C) 120°
D) 105°
E) 60°

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
59
Give the approximate bond angle for a molecule with an octahedral shape.
A) 109.5°
B) 60°
C) 145°
D) 107°
E) 90°
A) 109.5°
B) 60°
C) 145°
D) 107°
E) 90°

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
60
Use the molecular orbital diagram shown to determine which of the following is most stable. 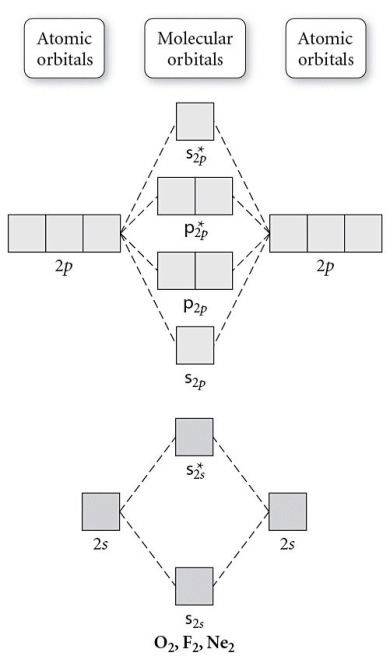
A) F2
B) F22⁺
C) Ne22⁺
D) O22⁺
E) F22⁻

A) F2
B) F22⁺
C) Ne22⁺
D) O22⁺
E) F22⁻

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
61
Determine the electron geometry (eg)and molecular geometry (mg)of the underlined carbon in CH3F.
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, mg = trigonal planar
C) eg = trigonal planar, mg = tetrahedral
D) eg = linear, mg = bent
E) eg = tetrahedral, mg = trigonal planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, mg = trigonal planar
C) eg = trigonal planar, mg = tetrahedral
D) eg = linear, mg = bent
E) eg = tetrahedral, mg = trigonal planar

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
62
Determine the electron geometry (eg)and molecular geometry (mg)of the underlined atom CH3OH.
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, eg = trigonal planar
C) eg = tetrahedral, mg = trigonal planar
D) eg = trigonal bipyramidal, mg = trigonal bipyramidal
E) eg = octahedral, mg = square planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, eg = trigonal planar
C) eg = tetrahedral, mg = trigonal planar
D) eg = trigonal bipyramidal, mg = trigonal bipyramidal
E) eg = octahedral, mg = square planar

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
63
Determine the electron geometry (eg)and molecular geometry (mg)of XeF2.
A) eg = trigonal bipyramidal, mg = trigonal planar
B) eg = linear, mg = linear
C) eg = tetrahedral, mg = tetrahdeal
D) eg = trigonal bipyramidal, mg = linear
E) eg = tetrahedral, mg = bent
A) eg = trigonal bipyramidal, mg = trigonal planar
B) eg = linear, mg = linear
C) eg = tetrahedral, mg = tetrahdeal
D) eg = trigonal bipyramidal, mg = linear
E) eg = tetrahedral, mg = bent

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
64
Determine the electron geometry (eg)and molecular geometry (mg)of the underlined atom H2CO.
A) eg = tetrahedral, mg = tetrahedral
B) eg = trigonal planar, eg = trigonal planar
C) eg = tetrahedral, mg = trigonal pyramidal
D) eg = trigonal bipyramidal, mg = trigonal pyramidal
E) eg = octahedral, mg = trigonal planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = trigonal planar, eg = trigonal planar
C) eg = tetrahedral, mg = trigonal pyramidal
D) eg = trigonal bipyramidal, mg = trigonal pyramidal
E) eg = octahedral, mg = trigonal planar

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
65
Using the VSEPR model,the electron-domain geometry of the central atom in ICl4- is
A) linear.
B) trigonal planar.
C) tetrahedral.
D) trigonal bipyramidal.
E) octahedral.
A) linear.
B) trigonal planar.
C) tetrahedral.
D) trigonal bipyramidal.
E) octahedral.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following best describes IBrCl-? It has a molecular geometry that is
A) linear with no lone pairs on the I atom.
B) linear with lone pairs on the I atom.
C) nonlinear with no lone pairs on the I atom.
D) nonlinear with lone pairs on the I atom.
A) linear with no lone pairs on the I atom.
B) linear with lone pairs on the I atom.
C) nonlinear with no lone pairs on the I atom.
D) nonlinear with lone pairs on the I atom.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
67
Determine the electron geometry (eg)and molecular geometry (mg)of the underlined atom CH3OCH3.
A) eg = bent, mg = tetrahedral
B) eg = linear, eg = bent
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = trigonal planar
E) eg = octahedral, mg = square planar
A) eg = bent, mg = tetrahedral
B) eg = linear, eg = bent
C) eg = tetrahedral, mg = bent
D) eg = trigonal bipyramidal, mg = trigonal planar
E) eg = octahedral, mg = square planar

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
68
What is the molecular geometry of SbF52-?
A) octahedral
B) seesaw
C) square pyramidal
D) trigonal planar
E) planar
A) octahedral
B) seesaw
C) square pyramidal
D) trigonal planar
E) planar

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
69
Determine the electron geometry (eg)and molecular geometry (mg)of HCN.
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, mg = trigonal planar
C) eg = trigonal planar, mg = bent
D) eg = linear, mg = linear
E) eg = trigonal planar, mg = trigonal planar
A) eg = tetrahedral, mg = tetrahedral
B) eg = linear, mg = trigonal planar
C) eg = trigonal planar, mg = bent
D) eg = linear, mg = linear
E) eg = trigonal planar, mg = trigonal planar

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
70
Determine the electron geometry (eg)and molecular geometry (mg)of BrF3.
A) eg = trigonal planar, mg = trigonal planar
B) eg = trigonal bipyramidal, mg = T-shape
C) eg = trigonal planar, mg = bent
D) eg = trigonal bipyramidal, mg = bent
E) eg = tetrahedral, mg = trigonal planar
A) eg = trigonal planar, mg = trigonal planar
B) eg = trigonal bipyramidal, mg = T-shape
C) eg = trigonal planar, mg = bent
D) eg = trigonal bipyramidal, mg = bent
E) eg = tetrahedral, mg = trigonal planar

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
71
The bond angle in H2Se is
A) 107°.
B) <104.5°.
C) 109.5°.
D) 190°.
E) 145°.
A) 107°.
B) <104.5°.
C) 109.5°.
D) 190°.
E) 145°.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
72
What is the molecular geometry of SeCl4?
A) seesaw
B) square planar
C) square pyramidal
D) tetrahedral
E) trigonal pyramidal
A) seesaw
B) square planar
C) square pyramidal
D) tetrahedral
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
73
Determine the electron geometry (eg)and molecular geometry (mg)of KrF4.
A) eg = tetrahedral, mg = square planar
B) eg = linear, eg = tetrahedral
C) eg = tetrahedral, mg = trigonal pyramidal
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar
A) eg = tetrahedral, mg = square planar
B) eg = linear, eg = tetrahedral
C) eg = tetrahedral, mg = trigonal pyramidal
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = square planar

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
74
What is the molecular geometry of NCl3?
A) T-shaped
B) tetrahedral
C) trigonal planar
D) trigonal pyramidal
E) octahedral
A) T-shaped
B) tetrahedral
C) trigonal planar
D) trigonal pyramidal
E) octahedral

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
75
What is the molecular geometry of BrF4 -?
A) seesaw
B) square planar
C) square pyramidal
D) pyramidal
E) trigonal bipyramidal
A) seesaw
B) square planar
C) square pyramidal
D) pyramidal
E) trigonal bipyramidal

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
76
Determine the electron geometry (eg)and molecular geometry (mg)of ICl2⁻.
A) eg = tetrahedral, mg = trigonal bipyramidal
B) eg = tetrahedral, mg = trigonal pyramidal
C) eg = trigonal bipyramidal, mg = linear
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = bent
A) eg = tetrahedral, mg = trigonal bipyramidal
B) eg = tetrahedral, mg = trigonal pyramidal
C) eg = trigonal bipyramidal, mg = linear
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = bent

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
77
Determine the electron geometry (eg)and molecular geometry (mg)of NCl3.
A) eg = tetrahedral, mg = linear
B) eg = linear, mg = tetrahedral
C) eg = trigonal planar, mg = tetrahedral
D) eg = linear, mg = linear
E) eg = tetrahedral, mg = trigonal pyramidal
A) eg = tetrahedral, mg = linear
B) eg = linear, mg = tetrahedral
C) eg = trigonal planar, mg = tetrahedral
D) eg = linear, mg = linear
E) eg = tetrahedral, mg = trigonal pyramidal

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
78
Using the VSEPR model,the molecular geometry of the central atom in SO3 is
A) linear.
B) trigonal planar.
C) tetrahedral.
D) bent.
E) trigonal pyramidal.
A) linear.
B) trigonal planar.
C) tetrahedral.
D) bent.
E) trigonal pyramidal.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
79
Using the VSEPR model,the molecular geometry of the central atom in N2O is
A) linear.
B) trigonal planar.
C) tetrahedral.
D) bent.
E) trigonal pyramidal.
A) linear.
B) trigonal planar.
C) tetrahedral.
D) bent.
E) trigonal pyramidal.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
80
The bond angle in NBr3 is
A) 107°.
B) 104.5°.
C) 120°.
D) 60°.
E) 95°.
A) 107°.
B) 104.5°.
C) 120°.
D) 60°.
E) 95°.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck



