Deck 7: Protein Function and Evolution
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/26
Play
Full screen (f)
Deck 7: Protein Function and Evolution
1
Which of the following reversible reactions of carbon dioxide explains how hemoglobin serves as a transporter of carbon dioxide?
A)reaction with the imidazole group of the distal histidine where oxygen would normally be bound
B)reaction with the carboxyl group of glutamic acid to form a carbonic acid anhydride
C)reaction with the N-terminal amino groups to form a carbamate
D)reaction with the hydroxyl group of serine to form a carbonate
E)none of the above
A)reaction with the imidazole group of the distal histidine where oxygen would normally be bound
B)reaction with the carboxyl group of glutamic acid to form a carbonic acid anhydride
C)reaction with the N-terminal amino groups to form a carbamate
D)reaction with the hydroxyl group of serine to form a carbonate
E)none of the above
C
2
Which of the following models of allosteric transitions best applies to hemoglobin?
A)sequential (KNF)model
B)concerted (MWC)model
C)tertiary two-state model
D)tense versus relaxed model
E)none of the above
A)sequential (KNF)model
B)concerted (MWC)model
C)tertiary two-state model
D)tense versus relaxed model
E)none of the above
C
3
Which of the following mutations in the coding strand of DNA would be the most likely to cause a serious mutation?
A)AGA CGA
B)ATA TTA
C)GAA AAA
D)TAA TGA
E)ACT AGT
A)AGA CGA
B)ATA TTA
C)GAA AAA
D)TAA TGA
E)ACT AGT
GAA AAA
4
Which of the following is true regarding the effectors of hemoglobin-oxygen binding?
A)an increase in blood pH will cause hemoglobin to bind more tightly to oxygen
B)increased CO2 from increased muscle activity will result in an increase in the R state of hemoglobin
C)increased Cl- will cause the formation of a salt bridge between two Lys residues,one on an subunit,the other on a subunit
D)the binding site of 2,3-BPG contains several Asp and Glu residues which are repelled by the similar charge,pushing the two subunits away from each other
E)all of the above
A)an increase in blood pH will cause hemoglobin to bind more tightly to oxygen
B)increased CO2 from increased muscle activity will result in an increase in the R state of hemoglobin
C)increased Cl- will cause the formation of a salt bridge between two Lys residues,one on an subunit,the other on a subunit
D)the binding site of 2,3-BPG contains several Asp and Glu residues which are repelled by the similar charge,pushing the two subunits away from each other
E)all of the above

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
5
Recent studies have shown that the H-bond between the distal histidine and oxygen molecule in myoglobin has a strength of ~ 15 kJ/mol,but in hemoglobin,the strength of the bond is only ~8 kJ/mol.What does this suggest about the differences between myoglobin and hemoglobin?
A)myoglobin binds oxygen more strongly than hemoglobin
B)hemoglobin binds oxygen more strongly than myoglobin
C)the iron in myoglobin is more easily oxidized than in hemoglobin
D)the iron in hemoglobin is more easily oxidized than in myoglobin
E)none of the above
A)myoglobin binds oxygen more strongly than hemoglobin
B)hemoglobin binds oxygen more strongly than myoglobin
C)the iron in myoglobin is more easily oxidized than in hemoglobin
D)the iron in hemoglobin is more easily oxidized than in myoglobin
E)none of the above

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following mutations is correctly defined?
A)silent: change of a single base in the non-coding intron region of a gene
B)missense: substitution of a single base results in a premature stop codon
C)nonsense: substitution of a single base results in a complete change of amino acid sequence
D)frameshift: deletion of a single base results in a single amino acid change
E)permissive: results in a protein that has greater thermodynamic stability
A)silent: change of a single base in the non-coding intron region of a gene
B)missense: substitution of a single base results in a premature stop codon
C)nonsense: substitution of a single base results in a complete change of amino acid sequence
D)frameshift: deletion of a single base results in a single amino acid change
E)permissive: results in a protein that has greater thermodynamic stability

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
7
In sickle cell hemoglobin,a Glu is mutated to a _______.This causes the formation of _______ between hemoglobin molecules,ultimately forming large aggregates.
A)Arg;salt bridges
B)Cys;disulfide bonds
C)Val;hydrophobic interactions
D)Pro;disrupted -helix,resulting in several H-bonds and salt bridges
E)none of the above
A)Arg;salt bridges
B)Cys;disulfide bonds
C)Val;hydrophobic interactions
D)Pro;disrupted -helix,resulting in several H-bonds and salt bridges
E)none of the above

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is a homotropic effector of hemoglobin-oxygen binding?
A)O2
B)H+
C)CO2
D)Cl-
E)2,3-bisphosphoglycerate (2,3-BPG)
A)O2
B)H+
C)CO2
D)Cl-
E)2,3-bisphosphoglycerate (2,3-BPG)

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
9
Based on the plot of oxygen saturation versus partial pressure of oxygen,which of the following statements is true? 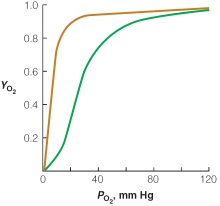
A)at oxygen pressure >100 mm Hg,hemoglobin dissociates into individual subunits so that it is able to bind oxygen similar to myoglobin
B)at typical resting capillary oxygen pressure of ~30 mm Hg,hemoglobin has only 1 of its 4 oxygen binding sites filled while myoglobin is nearly saturated with oxygen
C)under periods of extreme muscle exertion,capillary oxygen pressure can drop to 10 mm Hg,allowing release of ~90% of oxygen carried by hemoglobin
D)the structure of hemoglobin allows for complete release of oxygen in capillary beds at all times
E)none of the above

A)at oxygen pressure >100 mm Hg,hemoglobin dissociates into individual subunits so that it is able to bind oxygen similar to myoglobin
B)at typical resting capillary oxygen pressure of ~30 mm Hg,hemoglobin has only 1 of its 4 oxygen binding sites filled while myoglobin is nearly saturated with oxygen
C)under periods of extreme muscle exertion,capillary oxygen pressure can drop to 10 mm Hg,allowing release of ~90% of oxygen carried by hemoglobin
D)the structure of hemoglobin allows for complete release of oxygen in capillary beds at all times
E)none of the above

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
10
The ability of some bacteria to resist the cytotoxic effects of macrophages is most likely due to which of the following?
A)they have high levels of flavohemoglobin that allows for the destruction of nitric oxide,a potent cytotoxic compound produced by macrophages
B)they possess a protein called cytoglobin that causes macrophages to undergo apoptosis
C)they have high levels of a myoglobin-like protein that tightly binds oxygen,preventing proper activity of the macrophage
D)they possess the protein neuroglobin which enables them to specifically target nerve cells and thus evade macrophages
E)none of the above
A)they have high levels of flavohemoglobin that allows for the destruction of nitric oxide,a potent cytotoxic compound produced by macrophages
B)they possess a protein called cytoglobin that causes macrophages to undergo apoptosis
C)they have high levels of a myoglobin-like protein that tightly binds oxygen,preventing proper activity of the macrophage
D)they possess the protein neuroglobin which enables them to specifically target nerve cells and thus evade macrophages
E)none of the above

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following occurs when hemoglobin switches from the T (deoxy)state to the R (oxy)state?
A)the heme group goes from a slightly puckered conformation to a flat conformation
B)the ferrous ion is pulled into the plane of the heme group
C)the F8 (proximal)histidine rotates about 8 to better align with the ferrous ion
D)movement of the F8 histidine causes a shift in the F helix,thus weakening interactions with other subunits
E)all of the above
A)the heme group goes from a slightly puckered conformation to a flat conformation
B)the ferrous ion is pulled into the plane of the heme group
C)the F8 (proximal)histidine rotates about 8 to better align with the ferrous ion
D)movement of the F8 histidine causes a shift in the F helix,thus weakening interactions with other subunits
E)all of the above

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
12
Changes in hemoglobin's oxygen affinity are primarily the result of changes in the _________ structure of the protein.
A)primary
B)secondary
C)tertiary
D)quaternary
E)all of the above
A)primary
B)secondary
C)tertiary
D)quaternary
E)all of the above

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following interactions causes a change from the R state (oxy form)to the T state (deoxy form)of hemoglobin?
A)interactions between heme groups
B)protonation of the R-group of His146 on the subunit to allow formation of a salt bridge with Lys40 on the subunit
C)salt bridge formed by 1-Arg141 to the carboxylate of 2Asp126
D)protonation of the 1 subunit terminal carboxyl group disrupts a salt bridge with 2Lys 127
E)none of the above
A)interactions between heme groups
B)protonation of the R-group of His146 on the subunit to allow formation of a salt bridge with Lys40 on the subunit
C)salt bridge formed by 1-Arg141 to the carboxylate of 2Asp126
D)protonation of the 1 subunit terminal carboxyl group disrupts a salt bridge with 2Lys 127
E)none of the above

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
14
One would expect that defects in the globin genes would be so serious as to cause death in utero.Which of the following explains how some thalassemias are found in living people?
A)in -thalassemia,the use of the subunit allows individuals to live a normal lifespan
B)individuals who completely lack functional subunits can live if placed in a hypobaric chamber to induce hypoxia
C)since humans have 4 copies of the chain gene,effects are only seen when at least 3 of the genes are nonfunctional
D)individuals with nonfunctional chain genes can make 2 2 tetramers that function normally
E)none of the above
A)in -thalassemia,the use of the subunit allows individuals to live a normal lifespan
B)individuals who completely lack functional subunits can live if placed in a hypobaric chamber to induce hypoxia
C)since humans have 4 copies of the chain gene,effects are only seen when at least 3 of the genes are nonfunctional
D)individuals with nonfunctional chain genes can make 2 2 tetramers that function normally
E)none of the above

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
15
When oxygen is bound to myoglobin,the amino acid _____ is complexed to the iron ion of the heme group while _______ forms a hydrogen bound to the oxygen.
A)cysteine;serine
B)cysteine;histidine
C)serine;cysteine
D)histidine;histidine
E)histidine;cysteine
A)cysteine;serine
B)cysteine;histidine
C)serine;cysteine
D)histidine;histidine
E)histidine;cysteine

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following explains why the ferrous ion is not oxidized to the ferric state in the globin proteins even though free heme in solution is readily oxidized?
A)when bound to globins,the heme is always planar;when free in solution,heme adopts a non-planar configuration which allows the oxidation of the iron
B)the globin proteins provide a hydrophobic environment that prevents oxidation
C)coordination with the proximal histidine allows any oxidized iron to be rapidly reduced back to the ferrous state
D)since the oxygen is hydrogen bound to the distal histidine,if oxidation of iron does occur,the distal histidine allows for rapid reduction
E)none of the above
A)when bound to globins,the heme is always planar;when free in solution,heme adopts a non-planar configuration which allows the oxidation of the iron
B)the globin proteins provide a hydrophobic environment that prevents oxidation
C)coordination with the proximal histidine allows any oxidized iron to be rapidly reduced back to the ferrous state
D)since the oxygen is hydrogen bound to the distal histidine,if oxidation of iron does occur,the distal histidine allows for rapid reduction
E)none of the above

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
17
Myoglobin without a heme group would be considered a(n)_______.
A)apoprotein
B)holoprotein
C)apoenzyme
D)holoenzyme
E)none of the above
A)apoprotein
B)holoprotein
C)apoenzyme
D)holoenzyme
E)none of the above

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
18
Why do individuals who are heterozygous for sickle cell anemia have a resistance to malaria?
A)formation of hemoglobin aggregates makes red blood cells impervious to parasitic infection
B)shortened life span of red blood cells does not allow sufficient time for the parasite to mature in the red blood cell
C)diminished blood flow to the skin as a result of sickled red blood cells blocking capillaries prevents mosquitoes from actually transferring the malaria parasite to individuals
D)sickle cell hemoglobin fibers have both decreased O2 and CO2 carrying capacity;since CO2 is a mosquito attractant,diminished CO2 exhalation does not attract mosquitoes
E)none of the above
A)formation of hemoglobin aggregates makes red blood cells impervious to parasitic infection
B)shortened life span of red blood cells does not allow sufficient time for the parasite to mature in the red blood cell
C)diminished blood flow to the skin as a result of sickled red blood cells blocking capillaries prevents mosquitoes from actually transferring the malaria parasite to individuals
D)sickle cell hemoglobin fibers have both decreased O2 and CO2 carrying capacity;since CO2 is a mosquito attractant,diminished CO2 exhalation does not attract mosquitoes
E)none of the above

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
19
The binding of oxygen to hemoglobin is said to be _______________.
A)high affinity
B)low affinity
C)sigmoidal
D)cooperative
E)sequential
A)high affinity
B)low affinity
C)sigmoidal
D)cooperative
E)sequential

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
20
A typical Hill plot (log [YO2/(1- YO2)] vs log PO2)for hemoglobin-oxygen binding does not yield a straight line as it does for myoglobin.Which of the following is the best explanation for this?
A)at high oxygen pressures,hemoglobin is in a low oxygen affinity state
B)at low oxygen pressures,hemoglobin is in a high oxygen affinity state
C)hemoglobin undergoes a transition from low affinity state under low oxygen pressure to a high affinity state under high oxygen pressure
D)hemoglobin undergoes a transition from high affinity state under low oxygen pressure to a low affinity state under high oxygen pressure
E)none of the above
A)at high oxygen pressures,hemoglobin is in a low oxygen affinity state
B)at low oxygen pressures,hemoglobin is in a high oxygen affinity state
C)hemoglobin undergoes a transition from low affinity state under low oxygen pressure to a high affinity state under high oxygen pressure
D)hemoglobin undergoes a transition from high affinity state under low oxygen pressure to a low affinity state under high oxygen pressure
E)none of the above

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
21
2,3-Bisphosphoglycerate is known to interact with hemoglobin in the following ways:
carboxylate with Lys
C-2 phosphate with protonated His
C-3 phosphate with N-terminal amino acid
Using the structure of 2,3-bisphosphoglycerate,show these interactions and identify what type of interaction occurs.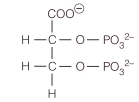
carboxylate with Lys
C-2 phosphate with protonated His
C-3 phosphate with N-terminal amino acid
Using the structure of 2,3-bisphosphoglycerate,show these interactions and identify what type of interaction occurs.


Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is responsible for the immense diversity of antibodies?
A)recombination of introns
B)recombination of exons
C)variable domain recombination
D)epitope recombination
E)none of the above
A)recombination of introns
B)recombination of exons
C)variable domain recombination
D)epitope recombination
E)none of the above

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
23
Using the following sequence from the coding strand of DNA,determine the amino acid sequence of the corresponding protein.Make three different mutations: (1)silent, (2)missense with little or no effect upon the protein structure (indicate the amino acid substitution)and (3)nonsense.
CATTTATATGAAAGC
CATTTATATGAAAGC

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
24
Given the following plot of oxygen binding versus pressure of O2 for myoglobin and hemoglobin, (1)draw a curve that shows what happens when the pH is increased to about 7.6 and (2)draw a curve that shows what happens when the pH is decreased to about 6.8. 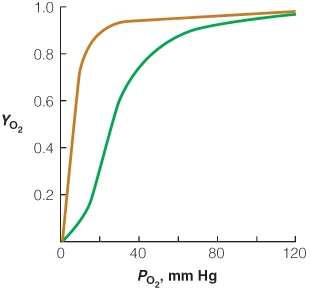


Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following methods could be used to produce a Fab fragment from an IgG?
A)treatment with reducing agent,isolation of the light chain,removal of reducing agent
B)treatment with reducing agent,isolation of the heavy chain,removal of reducing agent
C)treatment with papain,separation of the Fab fragment from the rest of the heavy chain
D)treatment with papain,separation of the Fab fragment from the rest of the light chain
E)none of the above
A)treatment with reducing agent,isolation of the light chain,removal of reducing agent
B)treatment with reducing agent,isolation of the heavy chain,removal of reducing agent
C)treatment with papain,separation of the Fab fragment from the rest of the heavy chain
D)treatment with papain,separation of the Fab fragment from the rest of the light chain
E)none of the above

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck
26
Which immunoglobulin is the only one to occur in a dimeric form?
A)IgA
B)IgD
C)IgE
D)IgG
E)IgM
A)IgA
B)IgD
C)IgE
D)IgG
E)IgM

Unlock Deck
Unlock for access to all 26 flashcards in this deck.
Unlock Deck
k this deck



