Deck 18: Organic Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/106
Play
Full screen (f)
Deck 18: Organic Chemistry
1
Organic compounds have to come from living things.
False
2
Alkynes are compounds with at least one triple bond.
True
3
All organic molecules contain carbon but not all carbon compounds are organic compounds.
True
4
Isomers are compounds with the same structures but different formulas.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
5
Alkanes undergo addition reactions.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
6
Carbon must always form four bonds.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
7
Hydrocarbon combustion reactions are highly endothermic - they absorb large amounts of heat.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
8
As the number of carbon atoms increases in n-alkanes,so does their boiling point.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
9
Alkenes and alkynes are unsaturated hydrocarbons.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
10
Combustion reactions always produce water and carbon monoxide.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
11
It was originally thought that organic compounds had to come from living things.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
12
Isomerism is very rare in organic compounds.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
13
Alkanes contain double and single bonds.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
14
The formula of n-octane is: CH3CH2CH2CH2CH2CH3.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
15
1-hexene and 1-hexyne are isomers.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
16
A noncyclical alkane hydrocarbon containing eight carbon atoms should also contain eighteen hydrogen atoms.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
17
The study of carbon-containing compounds and their reactions is called carbonyl chemistry.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
18
Hydrocarbons contain carbon,hydrogen and oxygen.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
19
The compound with the formula CH4 has four possible isomers.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
20
An ethyl group can be symbolized as -CH3.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
21
What chemical reaction was used by the German chemist Friedrich Wohler to synthesize urea for the first time?
A)evaporating urine
B)heating ammonium cyanate
C)heating ammonium cyanide
D)combining the elements carbon,hydrogen,oxygen,and nitrogen
E)none of the above
A)evaporating urine
B)heating ammonium cyanate
C)heating ammonium cyanide
D)combining the elements carbon,hydrogen,oxygen,and nitrogen
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
22
The formula for ethyl propyl ether is: CH3CH2OCH2CH2CH3.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
23
The generic condensed structural formula for carboxylic acids is RCOOH.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
24
When an alkene undergoes an addition reaction to form an alkane,a saturated compound has been converted to an unsaturated compound.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
25
Amines are nitrogen- containing organic compounds.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
26
CH3CH2CH2CH2CH2OH is named 5-pentanol.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
27
Aromatic compounds contain a benzene ring.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
28
What is the molecular geometry of the underlined carbon atom in the following compound, 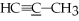
?
A)tetrahedral
B)trigonal planar
C)linear
D)bent
E)none of the above
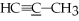
?
A)tetrahedral
B)trigonal planar
C)linear
D)bent
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
29
What is the molecular geometry of the underlined carbon atom in the following compound, 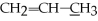
?
A)tetrahedral
B)trigonal planar
C)linear
D)bent
E)none of the above
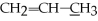
?
A)tetrahedral
B)trigonal planar
C)linear
D)bent
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
30
R- OH is an ether functional group.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
31
Aldehydes and ketones both contain a carbonyl group.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
32
The formula of propanoic acid is: CH2CH2COOH.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
33
The compound CH3NH2 can be classified as an amine.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
34
A polymer is a long chainlike compound containing a random arrangement of organic molecules called monomers.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
35
What is the molecular geometry of the underlined carbon atom in the following compound, 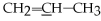
?
A)tetrahedral
B)trigonal planar
C)linear
D)bent
E)none of the above
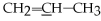
?
A)tetrahedral
B)trigonal planar
C)linear
D)bent
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
36
Production of the synthetic polymer "polyethylene" uses an alkene as a starting monomer unit.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
37
R-O-R is an ether functional group.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following statements about carbon is FALSE?
A)Carbon must always have four bonds.
B)Carbon can bond to itself.
C)Carbon can have either a tetrahedral,trigonal planar or linear geometry.
D)Carbon can form into linear,branched and cyclic compounds.
E)none of the above
A)Carbon must always have four bonds.
B)Carbon can bond to itself.
C)Carbon can have either a tetrahedral,trigonal planar or linear geometry.
D)Carbon can form into linear,branched and cyclic compounds.
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
39
The formula of methyl propanoate is: CH3CH2COOCH3.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
40
Esters are best known for their awful odors such as that associated with the smell of rotten fish.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following compounds is an alkene?
A)C2H6
B)C3H6
C)C4H6
D)C5H12
E)none of the above
A)C2H6
B)C3H6
C)C4H6
D)C5H12
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following structural formulas is an isomer to n-hexane?
A)C- C- C- C-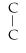 - C
- C
B)C-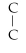 - C- C-
- C- C- 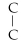
- C
C)C-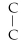 - C-
- C-
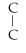 - C
- C
D)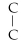
- C-

- C
E)none of the above
A)C- C- C- C-
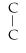 - C
- CB)C-
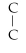 - C- C-
- C- C- 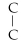
- C
C)C-
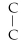 - C-
- C-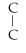 - C
- CD)
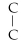
- C-

- C
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
43
Which type of hydrocarbon is classified as being saturated?
A)alkanes
B)alkenes
C)alkynes
D)all of these
E)none of the above
A)alkanes
B)alkenes
C)alkynes
D)all of these
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
44
Two compounds are considered as isomers if they have the
A)same molecular formula AND different structure.
B)same molecular formula AND same structure.
C)different molecular formula AND different structure.
D)different molecular formula AND same structure.
E)same molecular formula OR same structure.
A)same molecular formula AND different structure.
B)same molecular formula AND same structure.
C)different molecular formula AND different structure.
D)different molecular formula AND same structure.
E)same molecular formula OR same structure.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
45
Which is the name of C5H12?
A)n-butane
B)n-hexane
C)n-heptane
D)n-pentane
E)all of the above
A)n-butane
B)n-hexane
C)n-heptane
D)n-pentane
E)all of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is the formula for heptane?
A)C4H10
B)C3H8
C)C7H16
D)C2H6
E)all of the above
A)C4H10
B)C3H8
C)C7H16
D)C2H6
E)all of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following compounds is an alkyne?
A)C2H6
B)C3H6
C)C2H2
D)C5H12
E)none of the above
A)C2H6
B)C3H6
C)C2H2
D)C5H12
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
48
The prefix "but-" means that the base chain contains how many carbon atoms?
A)2
B)4
C)6
D)8
E)all of the above
A)2
B)4
C)6
D)8
E)all of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
49
How many isomers exist for the alkane whose molecular formula is C4H10?
A)1
B)2
C)3
D)4
E)none
A)1
B)2
C)3
D)4
E)none

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
50
Which formula represents butane?
A)C4H10
B)C3H8
C)C7H16
D)C2H6
E)all of the above
A)C4H10
B)C3H8
C)C7H16
D)C2H6
E)all of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
51
Which is the name of C6H14?
A)n-butane
B)n-hexane
C)n-heptane
D)n-pentane
E)all of the above
A)n-butane
B)n-hexane
C)n-heptane
D)n-pentane
E)all of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
52
A straight chain saturated hydrocarbon has eight carbon atoms.Its molecular formula is
A)C8H14.
B)C8H16.
C)C8H18.
D)C8H24.
E)none of the above
A)C8H14.
B)C8H16.
C)C8H18.
D)C8H24.
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
53
Based on the molecular formulas shown below,which compound shown below would NOT be classified as a noncyclical alkane?
A)C4H10
B)C3H8
C)C5H12
D)C12H24
E)C9H20
A)C4H10
B)C3H8
C)C5H12
D)C12H24
E)C9H20

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
54
Hydrocarbons are commonly used for:
A)fuel.
B)wax.
C)fabrics.
D)plastics.
E)all of the above
A)fuel.
B)wax.
C)fabrics.
D)plastics.
E)all of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
55
What will be the molecular geometry of an open-chain noncyclical hydrocarbon with the generic molecular formula CnH2n-2?
A)linear
B)tetrahedral
C)trigonal planar
D)trigonal pyramidal
E)none of the above
A)linear
B)tetrahedral
C)trigonal planar
D)trigonal pyramidal
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
56
Name the following alkane: CH3 - 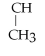
-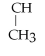
- CH2 - CH3
A)3-isopropylbutane
B)3,4-dimethylpentane
C)2,3-dimethylpentane
D)2-isopropylbutane
E)none of the above
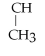
-
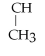
- CH2 - CH3
A)3-isopropylbutane
B)3,4-dimethylpentane
C)2,3-dimethylpentane
D)2-isopropylbutane
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
57
Name the following alkane: 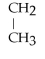
- CH2 -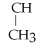
- CH2 - CH2 - CH3
A)1,3-dimethylhexane
B)4-methylheptane
C)2-propylpentane
D)1-methyl-1-propylbutane
E)none of the above
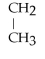
- CH2 -
- CH2 - CH2 - CH3
A)1,3-dimethylhexane
B)4-methylheptane
C)2-propylpentane
D)1-methyl-1-propylbutane
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following structural formulas is an isomer to n-nonane?
A)C- C- C- C-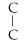
- C
B)C-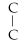
- C-
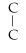
- C -
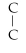
- C
C)C-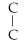
- C-
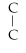
- C- C
D)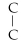
- C-
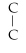
- C- C- C
E)none of the above
A)C- C- C- C-
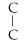
- C
B)C-
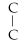
- C-
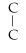
- C -
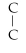
- C
C)C-
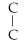
- C-
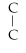
- C- C
D)
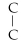
- C-
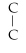
- C- C- C
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
59
Consider the following n-alkanes: CH4,C2H6,C3H8,C4H10
Which of these would you expect to have the highest boiling point?
A)CH4
B)C2H6
C)C3H8
D)C4H10
E)all n-alkanes have the same boiling point
Which of these would you expect to have the highest boiling point?
A)CH4
B)C2H6
C)C3H8
D)C4H10
E)all n-alkanes have the same boiling point

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
60
Which compound below is an alkane?
A)C4H10
B)CH3CH2CH3
C)C7H16
D)CH3CH2CH2CH2CH3
E)all of the above
A)C4H10
B)CH3CH2CH3
C)C7H16
D)CH3CH2CH2CH2CH3
E)all of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
61
What will be the major product(s)when C2H2 undergoes an addition reaction with Cl2?
A)C2HCl+ HCl
B)C2H2Cl2
C)C2Cl2 + H2
D)all of the above
E)none of the above
A)C2HCl+ HCl
B)C2H2Cl2
C)C2Cl2 + H2
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following compounds will undergo a substitution reaction?
A)C2H4
B)C2H2
C)C2H6
D)all of the above
E)none of the above
A)C2H4
B)C2H2
C)C2H6
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following functional groups represents an alcohol?
A)R-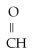
B)R -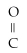
- R
C)R -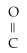
- OH
D)R -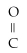
-O R
E)none of the above
A)R-
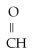
B)R -
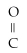
- R
C)R -
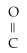
- OH
D)R -
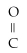
-O R
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
64
The combustion of alkanes,alkenes and alkynes produces:
A)water and carbon.
B)oxygen and hydrogen.
C)carbon dioxide and hydrogen.
D)hydrogen and water.
E)carbon dioxide and water.
A)water and carbon.
B)oxygen and hydrogen.
C)carbon dioxide and hydrogen.
D)hydrogen and water.
E)carbon dioxide and water.

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
65
What is the formula for propene?
A)C3H8
B)C3H6
C)C3H4
D)C3H3
E)none of the above
A)C3H8
B)C3H6
C)C3H4
D)C3H3
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
66
What will be the major product(s)when C2H4 undergoes an addition reaction with Cl2?
A)C2H3Cl2
B)C2H4Cl2
C)C2H2Cl2
D)all of the above
E)none of the above
A)C2H3Cl2
B)C2H4Cl2
C)C2H2Cl2
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
67
In naming disubstituted benzenes,"1,2" substitution is also known as:
A)ortho substitution.
B)meta substitution.
C)para substitution.
D)isomer substitution.
E)none of the above
A)ortho substitution.
B)meta substitution.
C)para substitution.
D)isomer substitution.
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
68
The reaction CH3CH3 + Br2 → CH3CH2Br + HBr would best be described as:
A)substitution.
B)combustion.
C)addition.
D)hydrogenation.
E)none of the above
A)substitution.
B)combustion.
C)addition.
D)hydrogenation.
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
69
What is the name of the following compound: 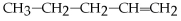
?
A)4-pentane
B)4-ene-pentane
C)1-pentene
D)2-pentane
E)none of the above
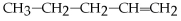
?
A)4-pentane
B)4-ene-pentane
C)1-pentene
D)2-pentane
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
70
What is the name of the following compound: 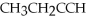
?
A)3-butene
B)1-butyne
C)2-butene
D)1-butene
E)none of the above
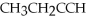
?
A)3-butene
B)1-butyne
C)2-butene
D)1-butene
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
71
The reaction CH2=CH2 + H2 → CH3CH3 would best be described as:
A)substitution.
B)combustion.
C)addition.
D)hydrogenation.
E)none of the above
A)substitution.
B)combustion.
C)addition.
D)hydrogenation.
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
72
What is the formula for propyne?
A)C3H8
B)C3H6
C)C3H4
D)C3H3
E)none of the above
A)C3H8
B)C3H6
C)C3H4
D)C3H3
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
73
Name the following alkane: CH3 - C = C - CH2 - CH3
| |
CH3 CH3
A)2-propyl-2-isobutene
B)1-propyl-2-butene
C)1,1,3-trimethyl-1-pentene
D)3,4-dimethyl-2-pentene
E)none of the above
| |
CH3 CH3
A)2-propyl-2-isobutene
B)1-propyl-2-butene
C)1,1,3-trimethyl-1-pentene
D)3,4-dimethyl-2-pentene
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
74
How many carbon atoms would be in the compound named chlorobenzene?
A)1
B)5
C)6
D)7
E)none of the above
A)1
B)5
C)6
D)7
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
75
Name the aromatic compound shown below: 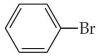
A)Phenyl bromide
B)Benzyl bromide
C)Bromobenzene
D)Phenol
E)none of the above

A)Phenyl bromide
B)Benzyl bromide
C)Bromobenzene
D)Phenol
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
76
The reaction CH2=CH2 + Br2 → CHBrCHBr would best be described as:
A)substitution.
B)combustion.
C)addition.
D)hydrogenation.
E)none of the above
A)substitution.
B)combustion.
C)addition.
D)hydrogenation.
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
77
What are the product(s)of: C2H6 + Cl2 ?
A)C2H6Cl2
B)C2H4Cl2 + H2
C)C2H5Cl + HCl
D)all of the above
E)none of the above
A)C2H6Cl2
B)C2H4Cl2 + H2
C)C2H5Cl + HCl
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
78
What are the products from the combustion of 2-methyl-3-ethyloctane?
A)variety of small hydrocarbons
B)carbon monoxide,carbon trioxide and water
C)carbon dioxide and water
D)carbon monoxide and water
E)none of the above
A)variety of small hydrocarbons
B)carbon monoxide,carbon trioxide and water
C)carbon dioxide and water
D)carbon monoxide and water
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
79
The compound named 2,3-dimethylpentane would have how many total number of carbons?
A)3
B)5
C)7
D)8
E)none of the above
A)3
B)5
C)7
D)8
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following compounds will undergo an addition reaction?
A)C3H8
B)C2H4
C)C2H6
D)all of the above
E)none of the above
A)C3H8
B)C2H4
C)C2H6
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 106 flashcards in this deck.
Unlock Deck
k this deck



