Deck 17: Radioactivity and Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/115
Play
Full screen (f)
Deck 17: Radioactivity and Nuclear Chemistry
1
A gamma ray is a high energy photon.
True
2
Radioactive particles can pass through matter.
True
3
Antoine-Henri Becquerel discovered X-rays.
False
4
When an atom emits a beta particle,its atomic number increases by one because it now has one additional proton.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
5
Nuclear equations do not need to be balanced since a new element forms.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
6
All elements with atomic numbers above bismuth are naturally radioactive.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
7
During beta decay,neutrons are converted to protons and electrons.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
8
When an atom emits an alpha particle,it becomes a different element.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
9
A gamma ray has no charge and no mass.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
10
Radioactivity is the emission of tiny,invisible radio signals by the nuclei of certain atoms.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
11
A nuclear reaction typically changes the identity of the element involved.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
12
A beta particle can also be called an electron.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
13
A positron particle comes from the decay of a proton into a neutron and a positron.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
14
Radioactive elements become stable isotopes after emitting one of the radioactive particles 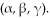
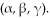

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
15
Madam Curie discovered two new elements.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
16
In a Geiger-Muller counter,radioactive particles pass through NaI which emits UV-Vis light.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
17
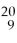
F contains 11 protons and 9 neutrons.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
18
Alpha particles have lower penetrating power than the other types of radioactive emissions..

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
19
The alpha particle (α)is: 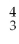
He.
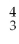
He.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
20
The symbol for a positron is 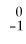
e.
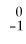
e.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
21
All unstable elements have the same rates of radioactive decay.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
22
Uranium-235 is capable of undergoing a fission chain reaction.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
23
The combination of two light nuclei to form a heavier one is known as nuclear fission.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
24
Nuclear fission reactions produce ten times more energy per gram than fusion reactions.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
25
The reaction 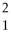
H +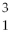
H →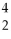
He +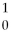
neutron is an example of nuclear fission.
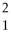
H +
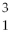
H →
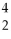
He +
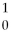
neutron is an example of nuclear fission.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
26
Nuclear power plants produce energy by fission reactions.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
27
The effort to build an atomic bomb during WWII was prompted by a fear that Germany was already working on nuclear weapons.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
28
The hydrogen isotope known as tritium contains three neutrons.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
29
Exposure to nuclear radioactivity can produce genetic defects in offspring.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
30
C-14 dating has been verified using old iron ore samples of known age.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
31
One danger of a nuclear power plant is that a chain reaction could produce a bomb.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
32
Nuclides that decay slowly have long half-lives.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
33
Phosphorous-32 has a half-life of 14.3 days while radon-222 has a half-life of 3.8 days,so phosphorous-32 is considered more active.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
34
A major natural source of radiation is due to the radon gas in our environment.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
35
Carbon-14 dating is not dependable when attempting to date objects that are more than 50,000 years old.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
36
The energy of the sun comes from fusion reactions.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
37
Carbon-14 dating is based on the fact that C-14 is continuously being made in the upper atmosphere.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
38
During the first half-life you lose half of the radioactive material,during the second half life you lose the remaining half.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
39
A fusion reaction emits large amounts of energy while a fission reaction absorbs large amounts of energy.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
40
A nuclide with a shorter half-life would be considered more active than a nuclide with a longer half-life.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
41
During the career of Marie Curie,she:
A)won a Nobel prize in chemistry.
B)won a Nobel prize in physics.
C)discovered the element polonium.
D)discovered the element radium.
E)all of the above
A)won a Nobel prize in chemistry.
B)won a Nobel prize in physics.
C)discovered the element polonium.
D)discovered the element radium.
E)all of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
42
What is the missing particle? 
A)positron
B)beta particle
C)alpha particle
D)gamma particle
E)none of the above

A)positron
B)beta particle
C)alpha particle
D)gamma particle
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
43
One method of nuclear medicine uses radioactive isotopes to scan specific regions of the body.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following statements about beta particles is FALSE?
A)The symbol is: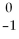
E.
B)Beta particles are created when neutrons become protons and electrons.
C)They have intermediate ionizing power.
D)They have intermediate penetrating power.
E)They are a safe form of radioactivity.
A)The symbol is:
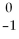
E.
B)Beta particles are created when neutrons become protons and electrons.
C)They have intermediate ionizing power.
D)They have intermediate penetrating power.
E)They are a safe form of radioactivity.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
45
What is the main product when 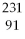
Pa undergoes alpha decay?
A)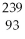
Pu
B)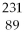
Ac
C)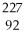
Ac
D)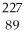
Ac
E)none of the above
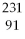
Pa undergoes alpha decay?
A)
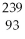
Pu
B)
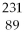
Ac
C)
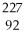
Ac
D)
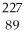
Ac
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following isotopes contains the most number of neutrons?
A)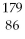
Rn
B)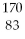
Bi
C)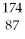
Fr
D)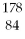
Po
E)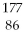
Rn
A)
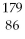
Rn
B)
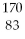
Bi
C)
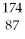
Fr
D)
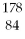
Po
E)
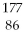
Rn

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
47
What happens to the mass number of a nucleus that emits an alpha particle?
A)It remains the same.
B)It decreases by two.
C)It decreases by four.
D)It increases by two.
E)It increases by four.
A)It remains the same.
B)It decreases by two.
C)It decreases by four.
D)It increases by two.
E)It increases by four.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
48
The first experiment that converted one element to another was performed in 1919 by Ernest Rutherford.An isotope of nitrogen was bombarded with a type of particle to get an oxygen atom as shown: 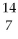
N + ? →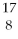
O +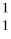
H
Which type of particle was used to bombard the nitrogen atom?
A)alpha
B)beta
C)positron
D)gamma
E)none of the above
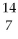
N + ? →
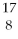
O +
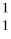
H
Which type of particle was used to bombard the nitrogen atom?
A)alpha
B)beta
C)positron
D)gamma
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
49
If the 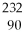
Th isotope emits an alpha particle,what would be the atomic number of the
Resulting atom?
A)90
B)88
C)92
D)228
E)236
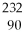
Th isotope emits an alpha particle,what would be the atomic number of the
Resulting atom?
A)90
B)88
C)92
D)228
E)236

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
50
What is the missing particle? 
A)positron
B)beta particle
C)alpha particle
D)gamma particle
E)none of the above

A)positron
B)beta particle
C)alpha particle
D)gamma particle
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
51
How many protons and neutrons are in 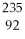
U?
A)92 p and 235 n
B)92 n and 235 p
C)92 n and 143 p
D)92 p and 143 n
E)none of the above
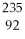
U?
A)92 p and 235 n
B)92 n and 235 p
C)92 n and 143 p
D)92 p and 143 n
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
52
What is the main product when 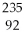
U undergoes alpha decay?
A)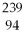
Am
B)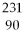
Th
C)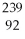
U
D)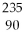
Th
E)none of the above
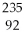
U undergoes alpha decay?
A)
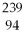
Am
B)
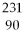
Th
C)
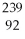
U
D)
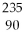
Th
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
53
How many protons and neutrons are in 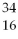
S?
A)16 p and 34 n
B)16 n and 34 p
C)16 n and 18 p
D)16 p and 18 n
E)none of the above
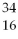
S?
A)16 p and 34 n
B)16 n and 34 p
C)16 n and 18 p
D)16 p and 18 n
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
54
A patient having radiotherapy uses radioactivity to kill infected cells while trying to minimize the effect on the healthy cells.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
55
Radioactive elements are used for:
A)medical diagnosis.
B)medical treatment.
C)determining age of fossils and rocks.
D)generating electricity.
E)all of the above
A)medical diagnosis.
B)medical treatment.
C)determining age of fossils and rocks.
D)generating electricity.
E)all of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
56
How many protons and neutrons are in 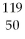
Sn?
A)50 p and 169 n
B)50 n and 69 p
C)50 n and 119 p
D)50 p and 69 n
E)none of the above
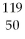
Sn?
A)50 p and 169 n
B)50 n and 69 p
C)50 n and 119 p
D)50 p and 69 n
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
57
Which statement about alpha particles is FALSE?
A)symbol is: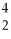
He
B)has high ionization power
C)has low penetrating power
D)are a harmless form of radiation
E)All of the above are true.
A)symbol is:
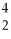
He
B)has high ionization power
C)has low penetrating power
D)are a harmless form of radiation
E)All of the above are true.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
58
Who discovered radioactivity?
A)Antoine Becquerel
B)Pierre Curie
C)Marie Curie
D)Ernest Rutherford
E)Benjamin Franklin
A)Antoine Becquerel
B)Pierre Curie
C)Marie Curie
D)Ernest Rutherford
E)Benjamin Franklin

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
59
How was radioactivity discovered?
A)Rutherford was investigating the structure of Au atoms and discovered alpha particles.
B)Marie Curie was investigating the existence of uranic rays that occurred in some minerals.
C)Becquerel was studying X-rays and phosphorescence to see if they were related.
D)Benjamin Franklin was studying the creation of electrical current from the ionization of gases due to radioactive particles.
E)none of the above
A)Rutherford was investigating the structure of Au atoms and discovered alpha particles.
B)Marie Curie was investigating the existence of uranic rays that occurred in some minerals.
C)Becquerel was studying X-rays and phosphorescence to see if they were related.
D)Benjamin Franklin was studying the creation of electrical current from the ionization of gases due to radioactive particles.
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
60
In a nuclear equation:
A)the daughter nuclide appears on the right-side of the arrow.
B)the sum of the atomic numbers on both sides must be equal.
C)the sum of the mass numbers on both sides must be equal.
D)all of the above
E)none of the above
A)the daughter nuclide appears on the right-side of the arrow.
B)the sum of the atomic numbers on both sides must be equal.
C)the sum of the mass numbers on both sides must be equal.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following is NOT a type of radioactive emission?
A)alpha rays
B)beta rays
C)gamma rays
D)positrons
E)All of the above are types of radioactive emissions.
A)alpha rays
B)beta rays
C)gamma rays
D)positrons
E)All of the above are types of radioactive emissions.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
62
Which type of emission has a negative charge?
A)alpha
B)beta
C)positron
D)gamma
E)none of the above
A)alpha
B)beta
C)positron
D)gamma
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
63
A Geiger-Muller counter detects radioactivity by:
A)developing film which is exposed by radioactive particles.
B)emission of light from a NaI crystal when radioactivity passes through the crystal.
C)ionization of argon gas in a chamber which produces an electrical signal.
D)analyzing the mass and velocity of each particle.
E)none of the above
A)developing film which is exposed by radioactive particles.
B)emission of light from a NaI crystal when radioactivity passes through the crystal.
C)ionization of argon gas in a chamber which produces an electrical signal.
D)analyzing the mass and velocity of each particle.
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
64
Which statement about positron emission is FALSE?
A)occurs when a proton is converted into a neutron and a positron
B)symbol is: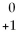
E
C)occurs with alpha and beta decay
D)alternative symbol is β+
E)All of the above are true.
A)occurs when a proton is converted into a neutron and a positron
B)symbol is:
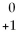
E
C)occurs with alpha and beta decay
D)alternative symbol is β+
E)All of the above are true.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following types of radiation has the highest penetrating power?
A)alpha particles
B)beta particles
C)gamma rays
D)positrons
E)none of the above
A)alpha particles
B)beta particles
C)gamma rays
D)positrons
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following nuclides will undergo beta decay to produce 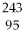
Am?
A)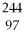
Bk
B)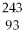
Np
C)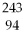
Pu
D)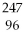
Cm
E)none of the above
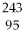
Am?
A)
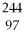
Bk
B)
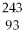
Np
C)
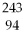
Pu
D)
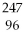
Cm
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
67
What happens to the atomic number of a nucleus that emits a beta particle?
A)It remains the same.
B)It increases by one.
C)It decreases by one.
D)It increases by two.
E)It decreases by two.
A)It remains the same.
B)It increases by one.
C)It decreases by one.
D)It increases by two.
E)It decreases by two.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
68
What is the missing particle? 
A)positron
B)beta particle
C)alpha particle
D)gamma particle
E)none of the above

A)positron
B)beta particle
C)alpha particle
D)gamma particle
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
69
The emitted particle with the mass of an electron but carrying a 1+ charge is named the:
A)proton.
B)proelectron.
C)positron.
D)plusion.
E)none of the above
A)proton.
B)proelectron.
C)positron.
D)plusion.
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
70
Radon-219 decays to radon-218 by releasing:
A)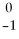
E.
B)gamma rays.
C)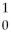
N.
D)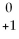
E.
E)none of the above
A)
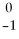
E.
B)gamma rays.
C)
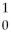
N.
D)
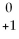
E.
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
71
What is the main product when 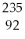
U undergoes beta decay?
A)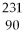
Th
B)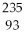
Np
C)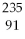
Pa
D)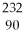
Th
E)none of the above
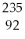
U undergoes beta decay?
A)
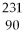
Th
B)
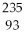
Np
C)
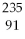
Pa
D)
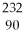
Th
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
72
What happens to the mass number of a nucleus that emits a positron?
A)It remains the same.
B)It increases by one.
C)It decreases by one.
D)It increases by two.
E)It decreases by two.
A)It remains the same.
B)It increases by one.
C)It decreases by one.
D)It increases by two.
E)It decreases by two.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
73
A scintillation counter detects radioactivity by:
A)developing film which is exposed by radioactive particles.
B)emission of light from a NaI crystal when radioactivity passes through the crystal.
C)ionization of argon gas in a chamber which produces an electrical signal.
D)analyzing the mass and velocity of each particle.
E)none of the above
A)developing film which is exposed by radioactive particles.
B)emission of light from a NaI crystal when radioactivity passes through the crystal.
C)ionization of argon gas in a chamber which produces an electrical signal.
D)analyzing the mass and velocity of each particle.
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
74
What is the main product when 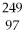
Bk emits a gamma ray?
A)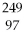
Bk
B)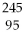
Am
C)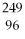
Cm
D)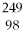
Cf
E)none of the above
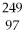
Bk emits a gamma ray?
A)
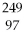
Bk
B)
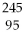
Am
C)
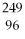
Cm
D)
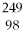
Cf
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
75
Which statement about gamma radiation is FALSE?
A)The symbol is: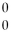
Γ.
B)They have low ionization energy.
C)They have high penetrating power.
D)They are high energy photons.
E)All of the above are true.
A)The symbol is:
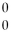
Γ.
B)They have low ionization energy.
C)They have high penetrating power.
D)They are high energy photons.
E)All of the above are true.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
76
How does the emission of a gamma particle effect the radioactive atom?
A)The atom has a lower amount of energy.
B)The atomic number decreases.
C)The atomic mass increases.
D)The atom gains energy for further radioactive particle emission.
E)All of the above are true.
A)The atom has a lower amount of energy.
B)The atomic number decreases.
C)The atomic mass increases.
D)The atom gains energy for further radioactive particle emission.
E)All of the above are true.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
77
What is the main product when 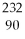
Th undergoes beta decay?
A)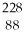
Ra
B)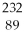
Ac
C)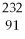
Pa
D)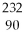
Th
E)none of the above
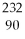
Th undergoes beta decay?
A)
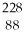
Ra
B)
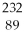
Ac
C)
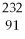
Pa
D)
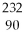
Th
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
78
What happens to the atomic number of a nucleus that emits a positron?
A)It remains the same.
B)It increases by one.
C)It decreases by one.
D)It increases by two.
E)It decreases by two.
A)It remains the same.
B)It increases by one.
C)It decreases by one.
D)It increases by two.
E)It decreases by two.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
79
What type of radioactive decay produces a daughter nuclide that is the same element as the parent nuclide?
A)alpha
B)beta
C)gamma
D)positron
E)none of the above
A)alpha
B)beta
C)gamma
D)positron
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
80
Which type of particle can be emitted by an unstable nucleus?
A)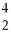
He
B)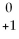
E
C)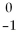
E
D)all of the above
E)none of the above
A)
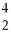
He
B)
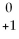
E
C)
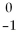
E
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck



