Deck 2: Chemistry Comes Alive
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/137
Play
Full screen (f)
Deck 2: Chemistry Comes Alive
1
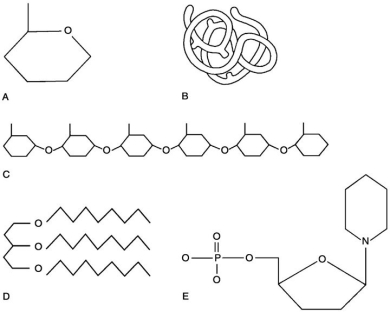
Figure 2.1
Using Figure 2.1, match the following:
A) C
B) B
C) E
D) D
E) A
Polysaccharide.
A
2
Match the following particles to the correct description:
A) Atom
B) Nuetron
C) Cation
D) Molecule
Electrically charged particle due to loss of an electron.
A) Atom
B) Nuetron
C) Cation
D) Molecule
Electrically charged particle due to loss of an electron.
C
3

Figure 2.1
Using Figure 2.1, match the following:
A) C
B) B
C) E
D) D
E) A
Monosaccharide.
E
4

Figure 2.1
Using Figure 2.1, match the following:
A) C
B) B
C) E
D) D
E) A
Nucleotide.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
5

Figure 2.1
Using Figure 2.1, match the following:
A) C
B) B
C) E
D) D
E) A
Polymer.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
6
Match the following chemical bonds to the correct description:
A) Nonpolar covalent bond
B) Polar covalent bond
C) Ionic bond
D) Hydrogen bond
A bond in which electrons are shared unequally.
A) Nonpolar covalent bond
B) Polar covalent bond
C) Ionic bond
D) Hydrogen bond
A bond in which electrons are shared unequally.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
7
Match the following chemical bonds to the correct description:
A) Nonpolar covalent bond
B) Polar covalent bond
C) Ionic bond
D) Hydrogen bond
A bond in which electrons are completely lost or gained by the atoms involved.
A) Nonpolar covalent bond
B) Polar covalent bond
C) Ionic bond
D) Hydrogen bond
A bond in which electrons are completely lost or gained by the atoms involved.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
8
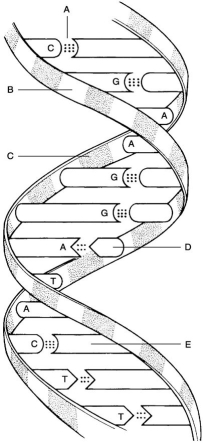 Figure 2.2
Figure 2.2Using Figure 2.2, match the following:
A) A
B) D
C) B
D) E
E) C
Hydrogen bonds.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
9
 Figure 2.2
Figure 2.2Using Figure 2.2, match the following:
A) A
B) D
C) B
D) E
E) C
Deoxyribose sugar.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
10
Match the following chemical bonds to the correct description:
A) Nonpolar covalent bond
B) Polar covalent bond
C) Ionic bond
D) Hydrogen bond
A bond in which electrons are shared equally.
A) Nonpolar covalent bond
B) Polar covalent bond
C) Ionic bond
D) Hydrogen bond
A bond in which electrons are shared equally.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
11

Figure 2.1
Using Figure 2.1, match the following:
A) C
B) B
C) E
D) D
E) A
Lipid.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
12
 Figure 2.2
Figure 2.2Using Figure 2.2, match the following:
A) A
B) D
C) B
D) E
E) C
Guanine.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
13

Figure 2.1
Using Figure 2.1, match the following:
A) C
B) B
C) E
D) D
E) A
Tertiary (protein)structure.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
14
Match the following particles to the correct description:
A) Atom
B) Nuetron
C) Cation
D) Molecule
Neutral subatomic particle.
A) Atom
B) Nuetron
C) Cation
D) Molecule
Neutral subatomic particle.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
15

Figure 2.1
Using Figure 2.1, match the following:
A) C
B) B
C) E
D) D
E) A
Functional protein.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
16
Match the following chemical bonds to the correct description:
A) Nonpolar covalent bond
B) Polar covalent bond
C) Ionic bond
D) Hydrogen bond
A type of bond important in tying different parts of the same molecule together into a three-dimensional structure.
A) Nonpolar covalent bond
B) Polar covalent bond
C) Ionic bond
D) Hydrogen bond
A type of bond important in tying different parts of the same molecule together into a three-dimensional structure.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
17
Match the following particles to the correct description:
A) Atom
B) Nuetron
C) Cation
D) Molecule
Smallest particle of an element that retains its properties.
A) Atom
B) Nuetron
C) Cation
D) Molecule
Smallest particle of an element that retains its properties.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
18
Match the following particles to the correct description:
A) Atom
B) Nuetron
C) Cation
D) Molecule
Combination of two or more atoms of the same element held together by chemical bonds.
A) Atom
B) Nuetron
C) Cation
D) Molecule
Combination of two or more atoms of the same element held together by chemical bonds.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
19
 Figure 2.2
Figure 2.2Using Figure 2.2, match the following:
A) A
B) D
C) B
D) E
E) C
Phosphate.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
20
 Figure 2.2
Figure 2.2Using Figure 2.2, match the following:
A) A
B) D
C) B
D) E
E) C
Thymine.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
21
Match the following:
A) Atomic symbol
B) Mass number of an element
C) Atomic number
Number of protons in an atom.
A) Atomic symbol
B) Mass number of an element
C) Atomic number
Number of protons in an atom.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
22
Match the following:
A) Weight
B) Mass
C) Matter
D) Energy
Although a man who weighs 175 pounds on Earth would be lighter on the moon and heavier on Jupiter,his ________ would not be different.
A) Weight
B) Mass
C) Matter
D) Energy
Although a man who weighs 175 pounds on Earth would be lighter on the moon and heavier on Jupiter,his ________ would not be different.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
23
The atomic weight is an average of the relative weights (mass numbers)of all the isotopes of an element.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
24
Match the following:
A) Mechanical energy
B) Chemical energy
C) Electrical energy
D) Radiant energy
Energy that travels in waves.Part of the electromagnetic spectrum.
A) Mechanical energy
B) Chemical energy
C) Electrical energy
D) Radiant energy
Energy that travels in waves.Part of the electromagnetic spectrum.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
25
Match the following:
A) Solutions
B) Suspensions
C) Colloids
Homogeneous,will not settle.
A) Solutions
B) Suspensions
C) Colloids
Homogeneous,will not settle.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
26
Match the following:
A) Mechanical energy
B) Chemical energy
C) Electrical energy
D) Radiant energy
When the bonds of ATP are broken,energy is released to do cellular work.
A) Mechanical energy
B) Chemical energy
C) Electrical energy
D) Radiant energy
When the bonds of ATP are broken,energy is released to do cellular work.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
27
Match the following:
A) Weight
B) Mass
C) Matter
D) Energy
Can be measured only by its effects on matter.
A) Weight
B) Mass
C) Matter
D) Energy
Can be measured only by its effects on matter.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
28
Match the following:
A) Atomic symbol
B) Mass number of an element
C) Atomic number
Combined number of protons and neutrons in an atom.
A) Atomic symbol
B) Mass number of an element
C) Atomic number
Combined number of protons and neutrons in an atom.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
29
Match the following:
A) Mechanical energy
B) Chemical energy
C) Electrical energy
D) Radiant energy
Represented by the flow of charged particles along a conductor,or the flow of ions across a membrane.
A) Mechanical energy
B) Chemical energy
C) Electrical energy
D) Radiant energy
Represented by the flow of charged particles along a conductor,or the flow of ions across a membrane.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
30
Match the following:
A) Solutions
B) Suspensions
C) Colloids
Will not scatter light.
A) Solutions
B) Suspensions
C) Colloids
Will not scatter light.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
31
Match the following:
A) Weight
B) Mass
C) Matter
D) Energy
Is a function of,and varies with,gravity.
A) Weight
B) Mass
C) Matter
D) Energy
Is a function of,and varies with,gravity.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
32
Match the following:
A) Mechanical energy
B) Chemical energy
C) Electrical energy
D) Radiant energy
Legs moving the pedals of a bicycle.
A) Mechanical energy
B) Chemical energy
C) Electrical energy
D) Radiant energy
Legs moving the pedals of a bicycle.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
33
Match the following:
A) Compound
B) Mixture
C) Element
Carbon.
A) Compound
B) Mixture
C) Element
Carbon.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
34
Match the following:
A) Solutions
B) Suspensions
C) Colloids
Heterogeneous,will settle.
A) Solutions
B) Suspensions
C) Colloids
Heterogeneous,will settle.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
35
Match the following:
A) Compound
B) Mixture
C) Element
Water.
A) Compound
B) Mixture
C) Element
Water.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
36
Match the following:
A) Weight
B) Mass
C) Matter
D) Energy
Anything that occupies space and has mass.
A) Weight
B) Mass
C) Matter
D) Energy
Anything that occupies space and has mass.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
37
Match the following:
A) Compound
B) Mixture
C) Element
Blood.
A) Compound
B) Mixture
C) Element
Blood.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
38
Match the following:
A) Atomic symbol
B) Mass number of an element
C) Atomic number
Usually,the first one or two letters of an element's name.
A) Atomic symbol
B) Mass number of an element
C) Atomic number
Usually,the first one or two letters of an element's name.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
39
Match the following:
A) Solutions
B) Suspensions
C) Colloids
Heterogeneous,will not settle.
A) Solutions
B) Suspensions
C) Colloids
Heterogeneous,will not settle.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
40
Match the following:
A) Compound
B) Mixture
C) Element
Dry ice (frozen carbon dioxide).
A) Compound
B) Mixture
C) Element
Dry ice (frozen carbon dioxide).

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
41
Glucose is an example of a monosaccharide.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
42
The acidity of a solution reflects the concentration of free hydrogen ions in the solution.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
43
Isotopes differ from each other only in the number of electrons the atom contains.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
44
Triglycerides are a poor source of stored energy.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
45
Chemical properties are determined primarily by neutrons.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
46
The lower the pH,the higher the hydrogen ion concentration.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
47
Buffers resist abrupt and large changes in the pH of body fluids by releasing or binding ions.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
48
About 60% to 80% of the volume of most living cells consists of organic compounds.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
49
Glycogen,the storage form of glucose,is primarily stored in skeletal muscle and liver cells.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
50
All organic compounds contain carbon.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
51
The fact that no chemical bonding occurs between the components of a mixture is the chief difference between mixtures and compounds.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
52
Mixtures are combinations of elements or compounds that are physically blended together but are not bound by chemical bonds.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
53
A charged particle is generally called an ion or electrolyte.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
54
A chemical bond is an energy relationship between outer electrons and neighboring atoms.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
55
It is the difference in the R group that makes each amino acid chemically unique.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
56
A dipeptide can be broken into two amino acids by dehydration synthesis.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
57
Covalent bonds are generally less stable than ionic bonds.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
58
Omega-3 fatty acids appear to decrease the risk of heart disease.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
59
Hydrogen bonds are too weak to bind atoms together to form molecules,but they do hold different parts of a single large molecule in a specific three-dimensional shape.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
60
The pH of body fluids must remain fairly constant for the body to maintain homeostasis.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
61
The single most abundant protein in the body is ________.
A) hemoglobin
B) collagen
C) DNA
D) glucose
A) hemoglobin
B) collagen
C) DNA
D) glucose

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
62
The genetic information is coded in DNA by the ________.
A) sequence of the nucleotides
B) three-dimensional structure of the double helix
C) arrangement of the histones
D) regular alteration of sugar and phosphate molecules
A) sequence of the nucleotides
B) three-dimensional structure of the double helix
C) arrangement of the histones
D) regular alteration of sugar and phosphate molecules

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
63
Salts are always ________.
A) double covalent compounds
B) single covalent compounds
C) ionic compounds
D) hydrogen bonded
A) double covalent compounds
B) single covalent compounds
C) ionic compounds
D) hydrogen bonded

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following does NOT characterize proteins?
A) Their function depends on their three-dimensional shape.
B) They may be denatured or coagulated by heat or acidity.
C) They appear to be the molecular carriers of coded hereditary information.
D) They have both functional and structural roles in the body.
A) Their function depends on their three-dimensional shape.
B) They may be denatured or coagulated by heat or acidity.
C) They appear to be the molecular carriers of coded hereditary information.
D) They have both functional and structural roles in the body.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
65
Choose the answer that best describes HCO3-.
A) a proton donor
B) a weak acid
C) a bicarbonate ion
D) common in the liver
A) a proton donor
B) a weak acid
C) a bicarbonate ion
D) common in the liver

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
66
Which statement about enzymes is FALSE?
A) Most enzymes can catalyze millions of reactions per minute.
B) Enzymes may be damaged by high temperature.
C) Enzymes may use coenzymes derived from vitamins or cofactors from metallic elements.
D) Enzymes require contact with substrate in order to assume their active form.
A) Most enzymes can catalyze millions of reactions per minute.
B) Enzymes may be damaged by high temperature.
C) Enzymes may use coenzymes derived from vitamins or cofactors from metallic elements.
D) Enzymes require contact with substrate in order to assume their active form.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following statements is FALSE?
A) Chemical reactions progress at a faster rate when the reacting particles are present in higher numbers.
B) Catalysts increase the rate of chemical reactions, sometimes while undergoing reversible changes in shape.
C) Larger particles move faster than smaller ones and thus collide more frequently and more forcefully.
D) Chemical reactions proceed more quickly at higher temperatures.
A) Chemical reactions progress at a faster rate when the reacting particles are present in higher numbers.
B) Catalysts increase the rate of chemical reactions, sometimes while undergoing reversible changes in shape.
C) Larger particles move faster than smaller ones and thus collide more frequently and more forcefully.
D) Chemical reactions proceed more quickly at higher temperatures.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
68
In general,the lipids that we refer to as oils have ________.
A) long fatty acid chains
B) a high water content
C) unsaturated fatty acids
D) saturated fatty acids
A) long fatty acid chains
B) a high water content
C) unsaturated fatty acids
D) saturated fatty acids

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
69
Carbohydrates are stored in the liver and skeletal muscles in the form of ________.
A) triglycerides
B) glycogen
C) glucose
D) cholesterol
A) triglycerides
B) glycogen
C) glucose
D) cholesterol

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
70
What structural level is represented by the coiling of the protein chain backbone into an alpha helix?
A) tertiary structure
B) secondary structure
C) primary structure
D) quaternary structure
A) tertiary structure
B) secondary structure
C) primary structure
D) quaternary structure

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following is a general function for a fibrous protein?
A) protein management
B) transport
C) structural framework
D) body defense
E) catalysis
A) protein management
B) transport
C) structural framework
D) body defense
E) catalysis

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
72
What happens in redox reactions?
A) the electron acceptor is oxidized
B) the organic substance that loses hydrogen is usually reduced
C) both decomposition and electron exchange occur
D) the reaction is uniformly reversible
A) the electron acceptor is oxidized
B) the organic substance that loses hydrogen is usually reduced
C) both decomposition and electron exchange occur
D) the reaction is uniformly reversible

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
73
Carbohydrates and proteins are built up from their basic building blocks by the ________.
A) addition of a carbon atom between each two units
B) removal of a water molecule between each two units
C) addition of a water molecule between each two units
D) removal of a carbon atom between each two units
A) addition of a carbon atom between each two units
B) removal of a water molecule between each two units
C) addition of a water molecule between each two units
D) removal of a carbon atom between each two units

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
74
The basic structural material of the body consists of ________.
A) proteins
B) lipids
C) nucleic acids
D) carbohydrates
A) proteins
B) lipids
C) nucleic acids
D) carbohydrates

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following statements is FALSE?
A) The pH of blood is slightly basic.
B) The more hydrogen ions in a solution, the more acidic the solution.
C) When acids and bases are mixed, they react with each other to form water and a salt.
D) When the hydrogen ion concentration decreases, the hydroxyl ion concentration also decreases.
A) The pH of blood is slightly basic.
B) The more hydrogen ions in a solution, the more acidic the solution.
C) When acids and bases are mixed, they react with each other to form water and a salt.
D) When the hydrogen ion concentration decreases, the hydroxyl ion concentration also decreases.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following does NOT describe enzymes?
A) Enzymes work by raising the energy of activation.
B) Some enzymes are purely protein.
C) Some enzymes are protein plus a cofactor.
D) Each enzyme is chemically specific.
A) Enzymes work by raising the energy of activation.
B) Some enzymes are purely protein.
C) Some enzymes are protein plus a cofactor.
D) Each enzyme is chemically specific.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following would be regarded as an organic molecule?
A) NaCl
B) H2O
C) CH4
D) NaOH
A) NaCl
B) H2O
C) CH4
D) NaOH

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
78
What is a chain of more than 50 amino acids called?
A) protein
B) triglyceride
C) nucleic acid
D) polysaccharide
A) protein
B) triglyceride
C) nucleic acid
D) polysaccharide

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following is the major positive ion outside cells?
A) hydrogen
B) magnesium
C) sodium
D) potassium
A) hydrogen
B) magnesium
C) sodium
D) potassium

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
80
The numbers listed represent the number of electrons in the first,second,and third energy levels,respectively.On this basis,which of the following is an unstable or reactive atom?
A) 2
B) 2, 8
C) 2, 8, 1
D) 2, 8, 8
A) 2
B) 2, 8
C) 2, 8, 1
D) 2, 8, 8

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck



