Deck 5: Chemical Bonding and States of Matter
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/90
Play
Full screen (f)
Deck 5: Chemical Bonding and States of Matter
1
Which is not a property of ionic compounds?
A) many are liquids at room temperature
B) high melting point
C) crystalline solid
D) melted ionic materials are conductors of electricity
A) many are liquids at room temperature
B) high melting point
C) crystalline solid
D) melted ionic materials are conductors of electricity
many are liquids at room temperature
2
Which substance is most likely to have covalent bonds between atoms?
A) AlCl3
B) KBr
C) MgF2
D) PCl3
A) AlCl3
B) KBr
C) MgF2
D) PCl3
PCl3
3
The shape of CH4 and CCl4 are best described as
A) tetrahedral.
B) pyramidal.
C) hexagonal.
D) octahedral.
A) tetrahedral.
B) pyramidal.
C) hexagonal.
D) octahedral.
tetrahedral.
4
Magnesium nitride is made up of magnesium ions and nitride ions. What is the expected formula of magnesium nitride?
A) MgN
B) Mg2N3
C) MgN2
D) Mg3N2
A) MgN
B) Mg2N3
C) MgN2
D) Mg3N2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
5
The compound between calcium and chlorine is calcium chloride and has which of the following formulas?
A) Cl2Ca3
B) Ca2Cl
C) CaCl2
D) ClCa2
A) Cl2Ca3
B) Ca2Cl
C) CaCl2
D) ClCa2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
6
Which one of the following shows the possible ions for iron?
A) Fe2+ and Fe4+
B) Fe+ and Fe2+
C) Fe2+ and Fe3+
D) Fe3+ and Fe+
A) Fe2+ and Fe4+
B) Fe+ and Fe2+
C) Fe2+ and Fe3+
D) Fe3+ and Fe+

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
7
What ion is likely to be formed by magnesium (Mg) in chemical reactions?
A) Mg+
B) Mg2+
C) Mg-
D) Mg2-
A) Mg+
B) Mg2+
C) Mg-
D) Mg2-

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
8
How many nonbonding electrons are in the water molecule?
A) 0
B) 2
C) 4
D) 6
A) 0
B) 2
C) 4
D) 6

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
9
A new element has the symbol Y and seven valence electrons. What is the maximum number of hydrogen atoms that can bond to Y?
A) 1
B) 4
C) 3
D) 7
A) 1
B) 4
C) 3
D) 7

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following pairs of atoms share electrons equally?
A) Cl and H
B) Mg and Cl
C) C and C
D) S and F
A) Cl and H
B) Mg and Cl
C) C and C
D) S and F

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following substances has polar covalent bonds?
A) CaF2
B) KBr
C) NH3
D) CH4
A) CaF2
B) KBr
C) NH3
D) CH4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following pairs of atoms form the most polar bond?
A) C and C
B) C and F
C) C and H
D) C and O
A) C and C
B) C and F
C) C and H
D) C and O

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
12
An ion contains 16 protons, 16 neutrons, and 18 electrons. Its charge is
a.
a.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
13
Washing soda, soda ash, and baking soda all contain which of the following groupings?
A) OH-
B) CO32-
C) PO43-
D) NO3-
A) OH-
B) CO32-
C) PO43-
D) NO3-

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
14
Which is expected to form a negative ion?
A) K
B) Ne
C) Na
D) F
A) K
B) Ne
C) Na
D) F

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
15
Of the following, which is the strongest bond?
A) C:C, single bond
B) C::C, double bond
C) C:::C, triple bond
D) All are of equal strength.
A) C:C, single bond
B) C::C, double bond
C) C:::C, triple bond
D) All are of equal strength.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
16
Which is not true about the ionic compound sodium chloride (NaCl)?
A) It is electrically neutral.
B) It was formed when electrons were shared.
C) It has properties different from the atoms from which it is formed.
D) It is a white crystalline solid.
A) It is electrically neutral.
B) It was formed when electrons were shared.
C) It has properties different from the atoms from which it is formed.
D) It is a white crystalline solid.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
17
If you take three balloons, blow them up and assemble them together, the shape you are most likely to observe is
A) linear.
B) tetrahedral.
C) pyramidal.
D) trigonal planar.
A) linear.
B) tetrahedral.
C) pyramidal.
D) trigonal planar.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
18
An ion contains 16 protons, 16 neutrons, and 18 electrons. Its charge is
A) 0
B) +2.
C) -2.
D) -18.
A) 0
B) +2.
C) -2.
D) -18.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following would be used to recognize an unsaturated hydrocarbon in its structural formula?
A) presence of a lone pair of electrons
B) multiple covalent bond
C) presence of carbon and hydrogen
D) fits the general formula CnH2n+2
A) presence of a lone pair of electrons
B) multiple covalent bond
C) presence of carbon and hydrogen
D) fits the general formula CnH2n+2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
20
Which substance has ionic bonds between atoms?
A) Na2O
B) NCl3
C) H2S
D) CO2
A) Na2O
B) NCl3
C) H2S
D) CO2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
21
Surface tension of water is illustrated by this phenomenon
A) moderating influence of lakes and oceans on climate.
B) cooling effect that occurs when water evaporates from moist skin.
C) beading of water droplets on the clean surface of a car's hood and roof.
D) expansion of water when it freezes.
A) moderating influence of lakes and oceans on climate.
B) cooling effect that occurs when water evaporates from moist skin.
C) beading of water droplets on the clean surface of a car's hood and roof.
D) expansion of water when it freezes.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
22
The cooling effect that occurs when water evaporates from moist skin is a consequence of this property of water
A) heat capacity.
B) surface tension.
C) heat of vaporization.
D) vapor pressure.
A) heat capacity.
B) surface tension.
C) heat of vaporization.
D) vapor pressure.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
23
Which statement is true about a glass of iced tea flavored with sugar and lemon juice?
A) Sugar is a solute.
B) Water is a solvent.
C) Lemon juice is a solute.
D) all of these are true
A) Sugar is a solute.
B) Water is a solvent.
C) Lemon juice is a solute.
D) all of these are true

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
24
When an ionic solid such as NaCl dissolves in water,
A) the Na+ and Cl- are changed to atoms.
B) the Na+ ions gather on one side of the container and the Cl- ions gather on the other side of the container.
C) Na+ and Cl- ions are evenly dispersed.
D) the beaker containing the solution becomes charged.
A) the Na+ and Cl- are changed to atoms.
B) the Na+ ions gather on one side of the container and the Cl- ions gather on the other side of the container.
C) Na+ and Cl- ions are evenly dispersed.
D) the beaker containing the solution becomes charged.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following has bond a angle of 180° between atoms?
A) CH4
B) SO3
C) H2O
D) CO2
A) CH4
B) SO3
C) H2O
D) CO2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
26
Most substances that have molecules with approximately the same weight as water molecules are gases at room temperature. Water is a liquid at room temperature because of
A) extensive hydrogen bonding between water molecules.
B) ionic bonding between hydrogen ions and oxide ions.
C) covalent bonding between hydrogen atoms and oxygen atoms.
D) the lack of motion of water molecules.
A) extensive hydrogen bonding between water molecules.
B) ionic bonding between hydrogen ions and oxide ions.
C) covalent bonding between hydrogen atoms and oxygen atoms.
D) the lack of motion of water molecules.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
27
When enough gas molecules stick together to form small droplets of liquid, the process is called
A) sublimation.
B) condensation.
C) deposition.
D) compression.
A) sublimation.
B) condensation.
C) deposition.
D) compression.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
28
There are ____ electrons in the covalent bond between C and O in CO.
A) 2
B) 4
C) 6
D) 8
A) 2
B) 4
C) 6
D) 8

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
29
Particles in the ____ state are in direct contact but are free to move.
A) gas
B) solid
C) liquid
D) plasma
A) gas
B) solid
C) liquid
D) plasma

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
30
When a spoonful of sugar is added to a glass of iced tea, it quickly dissolves. If no solid settles to the bottom, the resulting solution is
A) saturated.
B) unsaturated.
C) ionic.
D) normal.
A) saturated.
B) unsaturated.
C) ionic.
D) normal.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
31
Sublimation is the process where a
A) solid changes into a gas.
B) solid changes into a liquid.
C) liquid changes into a gas.
D) liquid changes into a solid.
A) solid changes into a gas.
B) solid changes into a liquid.
C) liquid changes into a gas.
D) liquid changes into a solid.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
32
A neutral atom can be converted to a negative ion if it
A) gains electrons.
B) loses electrons.
C) gains protons.
D) loses protons.
A) gains electrons.
B) loses electrons.
C) gains protons.
D) loses protons.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
33
Gases
A) exert a pressure.
B) are compressible.
C) are miscible, they mix with other gases in any proportion.
D) all of these
A) exert a pressure.
B) are compressible.
C) are miscible, they mix with other gases in any proportion.
D) all of these

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
34
The dispersal of odors is partially due to a property of gases known as
A) diffusion.
B) miscibility.
C) expansion.
D) volatility.
A) diffusion.
B) miscibility.
C) expansion.
D) volatility.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
35
An atom has an electronic arrangement of 2-8-8-2. This atom is most likely to
A) form a +2 ion
B) bond covalently to nonmetals
C) form a -2 ion
D) bond covalently to metals
A) form a +2 ion
B) bond covalently to nonmetals
C) form a -2 ion
D) bond covalently to metals

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following molecules has a double bond?
A) CH2CH2
B) NH3
C) CH3CH3
D) H2O
A) CH2CH2
B) NH3
C) CH3CH3
D) H2O

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
37
How do aqueous electrolytes conduct electricity?
A) Dissolved positive ions move to positive electrodes.
B) Electrons migrate from one electrode to the other electrode.
C) Ions pass electrons from ion to ion.
D) Dissolved ions move to oppositely charged electrodes.
A) Dissolved positive ions move to positive electrodes.
B) Electrons migrate from one electrode to the other electrode.
C) Ions pass electrons from ion to ion.
D) Dissolved ions move to oppositely charged electrodes.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
38
The relation between gas pressure and volume was discovered by
A) Robert Boyle.
B) G. N. Lewis.
C) Marie Curie.
D) Rutherford.
A) Robert Boyle.
B) G. N. Lewis.
C) Marie Curie.
D) Rutherford.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
39
Liquids have a
A) fixed volume and no definite shape.
B) fixed shape and no volume.
C) no definite shape and no volume.
D) fixed shape and fixed volume.
A) fixed volume and no definite shape.
B) fixed shape and no volume.
C) no definite shape and no volume.
D) fixed shape and fixed volume.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
40
What particles make up the crystal in a grain of table salt (sodium chloride)?
A) atoms of sodium and chlorine
B) molecules of sodium and chlorine
C) sodium ions and chloride ions
D) molecules of sodium chloride
A) atoms of sodium and chlorine
B) molecules of sodium and chlorine
C) sodium ions and chloride ions
D) molecules of sodium chloride

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
41
The normal boiling point of water is
A) what you measure in a laboratory.
B) independent of atmospheric pressure.
C) the one measured at 760 mm pressure (1 atm).
D) 273 K.
A) what you measure in a laboratory.
B) independent of atmospheric pressure.
C) the one measured at 760 mm pressure (1 atm).
D) 273 K.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
42
Which molecule would be expected to exhibit the least hydrogen bonding?
A) H-F
B) H-O-H
C) NH3
D) CH4
A) H-F
B) H-O-H
C) NH3
D) CH4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
43
Identify the saturated hydrocarbon listed below.
A) C3H8
B) C4H8
C) C2H4
D) C6H6
A) C3H8
B) C4H8
C) C2H4
D) C6H6

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following molecules has only single bonds?
A) CH2CH2
B) CH3CH3
C) CO2
D) CHCH
A) CH2CH2
B) CH3CH3
C) CO2
D) CHCH

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
45
Which factor causes more gas to dissolve in a liquid?
A) size of the container opening
B) nature of the container (that is, glass versus metal)
C) increasing the pressure
D) increasing the temperature
A) size of the container opening
B) nature of the container (that is, glass versus metal)
C) increasing the pressure
D) increasing the temperature

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
46
Which is not a physical property of water?
A) solid is less dense than liquid at normal freezing point
B) relatively high heat capacity per unit of weight
C) relatively low heat of vaporization
D) relatively large surface tension
A) solid is less dense than liquid at normal freezing point
B) relatively high heat capacity per unit of weight
C) relatively low heat of vaporization
D) relatively large surface tension

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
47
Which atom exists as a diatomic molecule in nature?
A) carbon
B) bromine
C) sulfur
D) phosphorous
A) carbon
B) bromine
C) sulfur
D) phosphorous

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the states of matter has particles in fixed positions and touching one another?
A) solid
B) liquid
C) plasma
D) gas
A) solid
B) liquid
C) plasma
D) gas

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
49
What is name of the following compound NH4NO3?
A) ammonia nitrite
B) ammonium nitrite
C) nitrogen hydride nitrite
D) ammonium nitrate
A) ammonia nitrite
B) ammonium nitrite
C) nitrogen hydride nitrite
D) ammonium nitrate

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
50
The normal boiling point of a liquid is
A) high for volatile liquids.
B) high for nonvolatile liquids.
C) low for nonvolatile liquids.
D) The same for all liquids, that is what normal means.
A) high for volatile liquids.
B) high for nonvolatile liquids.
C) low for nonvolatile liquids.
D) The same for all liquids, that is what normal means.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
51
What is the expected formula for a reaction between aluminum and oxygen?
A) AlO
B) Al2O3
C) AlO2
D) Al3O2
A) AlO
B) Al2O3
C) AlO2
D) Al3O2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
52
How many resonance structures can be drawn for a nitrate ion?
A) 0
B) 1
C) 2
D) 3
A) 0
B) 1
C) 2
D) 3

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
53
What is the charge on an ion with 7 protons, 7 neutrons, and 10 electrons?
A) 0
B) +3
C) -3
D) -7
A) 0
B) +3
C) -3
D) -7

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
54
Which element has the highest electronegativity?
A) H
B) O
C) N
D) C
A) H
B) O
C) N
D) C

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
55
How many nonbonding electrons are found on the central atom of sulfur dioxide?
A) 0
B) 2
C) 4
D) 6
A) 0
B) 2
C) 4
D) 6

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
56
What is the maximum number of hydrogen bonds that one water molecule can form to its neighbors in an ice crystal?
A) 0
B) 2
C) 4
D) infinite number
A) 0
B) 2
C) 4
D) infinite number

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following elements can form a cation?
A) K
B) Ne
C) O
D) F
A) K
B) Ne
C) O
D) F

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
58
Which is not characteristic of liquids?
A) freezing point
B) crystallization
C) boiling point
D) All of these are characteristic of liquids.
A) freezing point
B) crystallization
C) boiling point
D) All of these are characteristic of liquids.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
59
What is the electron pair geometry for silicon dioxide?
A) linear
B) bent
C) trigonal pyramidal
D) tetrahedral
A) linear
B) bent
C) trigonal pyramidal
D) tetrahedral

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is an ionic compound?
A) CO2
B) NH3
C) CaO
D) C3H8
A) CO2
B) NH3
C) CaO
D) C3H8

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
61
Consider the image below which shows a model of the compound known as hexane. The large spheres represent carbon and the smaller spheres represent hydrogen. 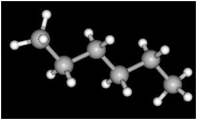
The total number of valence electrons in hexane is_________.

The total number of valence electrons in hexane is_________.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
62
Positive ions are smaller than the atoms for which they form.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
63
Consider the following Lewis dot structure for ozone, O3. 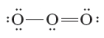 Based on these structures, what is the shape of this molecule?
Based on these structures, what is the shape of this molecule?
A) linear
B) bent
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal
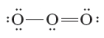 Based on these structures, what is the shape of this molecule?
Based on these structures, what is the shape of this molecule?A) linear
B) bent
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
64
The following images represent the process termed freezing.  changes to
changes to 
 changes to
changes to 

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following molecules might have the shape shown below? 
A) AlCl3
B) CCl4
C) SO3
D) TiCl4

A) AlCl3
B) CCl4
C) SO3
D) TiCl4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
66
How much heat is required to melt 1 kg of water (heat of melting = 80 cal/g)
A) -80 cal
B) +80 cal
C) -80000 cal
D) +80000 cal
A) -80 cal
B) +80 cal
C) -80000 cal
D) +80000 cal

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
67
Phosphorus has 3 valence electrons.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
68
What is the solvent in a solution of sodium chloride?
A) sodium chloride
B) sodium
C) oxygen
D) water
A) sodium chloride
B) sodium
C) oxygen
D) water

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
69
Consider the following two Lewis dot structures for ozone. These two structures are termed resonance structures. 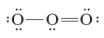
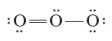
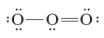
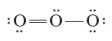

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
70
The following image shows two molecules of sulfur dioxide. 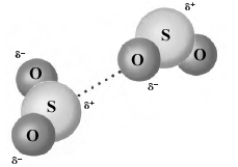 Which of the following describes the type of bonding present?
Which of the following describes the type of bonding present?
A) ionic bonding
B) polar covalent bonding
C) nonpolar covalent bonding
D) hydrogen bonding
 Which of the following describes the type of bonding present?
Which of the following describes the type of bonding present?A) ionic bonding
B) polar covalent bonding
C) nonpolar covalent bonding
D) hydrogen bonding

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following is a nonpolar molecule with a polar covalent bond?
A) HCl
B) NH3
C) H2O
D) CO2
A) HCl
B) NH3
C) H2O
D) CO2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
72
Choose the statement that best explains why the boiling point of ICl (97 °C) is higher than the boiling point of Br2 (59 °C),
A) ICl is an ionic compound, while Br2 is a covalent compound.
B) There is hydrogen bonding in ICl, but not in Br2.
C) ICl is polar while Br2 is nonpolar.
D) ICl has a bent shape while Br2 is linear.
A) ICl is an ionic compound, while Br2 is a covalent compound.
B) There is hydrogen bonding in ICl, but not in Br2.
C) ICl is polar while Br2 is nonpolar.
D) ICl has a bent shape while Br2 is linear.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
73
Consider the image below which shows a model of the compound known as hexane. The large spheres represent carbon and the smaller spheres represent hydrogen. 
The total number of single bonds in hexane is__________.

The total number of single bonds in hexane is__________.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
74
What is the molecular geometry of carbon tetrachloride?
A) tetrahedral
B) trigonal pyramidal
C) bent
D) trigonal planar
A) tetrahedral
B) trigonal pyramidal
C) bent
D) trigonal planar

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
75
Negative ions are approximately the same size as the atoms from which they form.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
76
Consider the following image.  What shape is represented?
What shape is represented?
A) linear
B) bent
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal
 What shape is represented?
What shape is represented?A) linear
B) bent
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
77
The dotted line in the following images shows the formation of hydrogen bonds. 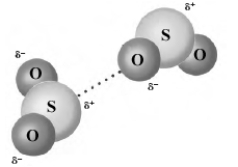


Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
78
A binary ionic compound generally consists of a metal and a nonmetal combination.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following molecules has a triple bond?
A) CO2
B) NH3
C) Cl2
D) N2
A) CO2
B) NH3
C) Cl2
D) N2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck



