Deck 7: Chemical Quantities and Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/114
Play
Full screen (f)
Deck 7: Chemical Quantities and Reactions
1
Calculate the molar mass of potassium chloride,KCl.
A)74.55 g/mole
B)54.55 g/mole
C)6.745 g/mole
D)67.45 g/mole
E)19.00 g/mole
A)74.55 g/mole
B)54.55 g/mole
C)6.745 g/mole
D)67.45 g/mole
E)19.00 g/mole
74.55 g/mole
2
0.400 mole of sucrose, C12H22O11 ,contains ________ moles of C.
A)12
B)6.02 × 1023
C)5.02 × 1023
D)2.89 × 1024
E)4.80
A)12
B)6.02 × 1023
C)5.02 × 1023
D)2.89 × 1024
E)4.80
4.80
3
One mole of copper(II)sulfate,CuSO4 ,contains ________ O atoms.
A)4
B)6.02 × 1023
C)1.51 × 1023
D)2.41 × 1023
E)2.41 × 1024
A)4
B)6.02 × 1023
C)1.51 × 1023
D)2.41 × 1023
E)2.41 × 1024
2.41 × 1024
4
One mole of neon atoms has a mass of ________.
A)6.02 × 1023 g
B)14.01 g
C)10.01 g
D)20.18 g
E)22.99 g
A)6.02 × 1023 g
B)14.01 g
C)10.01 g
D)20.18 g
E)22.99 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
5
0.400 mole of sucrose, C12H22O11
,contains ________ C atoms.
A)12
B)6.02 × 1023
C)5.02 × 1022
D)2.89 × 1024
E)4.80
,contains ________ C atoms.
A)12
B)6.02 × 1023
C)5.02 × 1022
D)2.89 × 1024
E)4.80

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
6
One mole of copper(II)sulfate,CuSO4 ,contains ________ moles of O.
A)4
B)6.02 × 1023
C)1.51 × 1023
D)2.41 × 1023
E)2.41 × 1024
A)4
B)6.02 × 1023
C)1.51 × 1023
D)2.41 × 1023
E)2.41 × 1024

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
7
What is the molar mass of sucrose ( C12H22O11 )?
A)29.02 g/mole
B)50.23 g/mole
C)210.0 g/mole
D)342.3 g/mole
E)182.0 g/mole
A)29.02 g/mole
B)50.23 g/mole
C)210.0 g/mole
D)342.3 g/mole
E)182.0 g/mole

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
8
0.100 mole of lithium has a mass of ________.
A)3.00 g
B)0.300 g
C)6.94 g
D)0.694 g
E)0.700 g
A)3.00 g
B)0.300 g
C)6.94 g
D)0.694 g
E)0.700 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
9
The molar mass of potassium is ________.
A)19.00 g/mole
B)31.00 g/mole
C)6.02 × 1023 g/mole
D)39.10 g/mole
E)15.02 g/mole
A)19.00 g/mole
B)31.00 g/mole
C)6.02 × 1023 g/mole
D)39.10 g/mole
E)15.02 g/mole

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
10
Avogadro's number is the number of ________.
A)particles in 1 mole of a substance
B)amu in 1 mole of a substance
C)grams in 1 mole of a substance
D)moles in 6.02 × 1023 g of an element
E)moles in 6.02 × 1023 amu of an element
A)particles in 1 mole of a substance
B)amu in 1 mole of a substance
C)grams in 1 mole of a substance
D)moles in 6.02 × 1023 g of an element
E)moles in 6.02 × 1023 amu of an element

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
11
What is the molar mass of Mg3(PO4)2 ,a substance formerly used in medicine as an antacid?
A)71.28 g/mole
B)118.3 g/mole
C)150.3 g/mole
D)214.3 g/mole
E)262.9 g/mole
A)71.28 g/mole
B)118.3 g/mole
C)150.3 g/mole
D)214.3 g/mole
E)262.9 g/mole

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
12
The molar mass of C3H8O2 is ________.
A)76.09 g/mole
B)60.09 g/mole
C)29.02 g/mole
D)69.02 g/mole
E)52.01 g/mole
A)76.09 g/mole
B)60.09 g/mole
C)29.02 g/mole
D)69.02 g/mole
E)52.01 g/mole

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
13
The molar mass of calcium hydroxide, Ca(OH)2 ,is ________.
A)58.09 g/mole
B)57.09 g/mole
C)74.10 g/mole
D)114.2 g/mole
E)54.02 g/mole
A)58.09 g/mole
B)57.09 g/mole
C)74.10 g/mole
D)114.2 g/mole
E)54.02 g/mole

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
14
One mole of particles of any substance contains how many particles?
A) 106 particles
B)3 × 10-10 particles
C)3 × 1010 particles
D)6.02 × 1023 particles
E)6.02 × 10 - 23 particles
A) 106 particles
B)3 × 10-10 particles
C)3 × 1010 particles
D)6.02 × 1023 particles
E)6.02 × 10 - 23 particles

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
15
What is the molar mass of sodium phosphate, Na3PO4 ?
A)69.96 g/mole
B)118.0 g/mole
C)163.9 g/mole
D)226.1 g/mole
E)354.0 g/mole
A)69.96 g/mole
B)118.0 g/mole
C)163.9 g/mole
D)226.1 g/mole
E)354.0 g/mole

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
16
What is the molar mass of copper(II)sulfate,CuSO4 ?
A)16.00 g/mole
B)63.60 g/mole
C)111.6 g/mole
D)159.6 g/mole
E)319.2 g/mole
A)16.00 g/mole
B)63.60 g/mole
C)111.6 g/mole
D)159.6 g/mole
E)319.2 g/mole

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
17
How many moles of water, H2O,are present in 75.0 g H2O?
A)4.41 moles
B)4.16 moles
C)75.0 moles
D)7.50 moles
E)1.35 × 1023 moles
A)4.41 moles
B)4.16 moles
C)75.0 moles
D)7.50 moles
E)1.35 × 1023 moles

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
18
The molar mass of helium is ________.
A)1.000 g
B)2.002 g
C)3.003 g
D)4.003 g
E)8.006 g
A)1.000 g
B)2.002 g
C)3.003 g
D)4.003 g
E)8.006 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
19
Calculate the molar mass of magnesium chloride,MgCl2 .
A)24.31 g/mole
B)95.21 g/mole
C)125.9 g/mole
D)59.76 g/mole
E)70.90 g/mole
A)24.31 g/mole
B)95.21 g/mole
C)125.9 g/mole
D)59.76 g/mole
E)70.90 g/mole

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
20
How many moles of carbon atoms are there in 0.500 mole of C2H6 ?
A)0.500 mole
B)1.00 mole
C)3.00 moles
D)6.02 × 1023 moles
E)4.00 moles
A)0.500 mole
B)1.00 mole
C)3.00 moles
D)6.02 × 1023 moles
E)4.00 moles

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
21
Pentane ( C5H12 )reacts with oxygen ( O2 )to form carbon dioxide ( CO2 )and water ( H2O)according to the following reaction.Answer the following question(s)about this reaction. C5H12 + ?O2 → ?CO2 + ?H2O
In the following reaction,what is the correct coefficient for aluminum chloride? Al + Cl2 → AlCl3
A)1
B)2
C)3
D)4
E)5
In the following reaction,what is the correct coefficient for aluminum chloride? Al + Cl2 → AlCl3
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
22
What is the coefficient of hydrogen, H2 ,when the following equation is balanced? Al + H2SO4 → Al2(SO4)3+ ?H2
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
23
Pentane ( C5H12 )reacts with oxygen ( O2 )to form carbon dioxide ( CO2 )and water ( H2O)according to the following reaction.Answer the following question(s)about this reaction. C5H12 + ?O2 → ?CO2 + ?H2O
In this reaction,what is the coefficient for calcium oxide? CaO + CO2 → CaCO3
A)1
B)2
C)3
D)4
E)5
In this reaction,what is the coefficient for calcium oxide? CaO + CO2 → CaCO3
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
24
Pentane ( C5H12 )reacts with oxygen ( O2 )to form carbon dioxide ( CO2 )and water ( H2O)according to the following reaction.Answer the following question(s)about this reaction. C5H12 + ?O2 → ?CO2 + ?H2O
In this reaction,what is the correct coefficient for sodium chloride? Pb(NO3)2 + ? NaCl → PbCl2 + ? NaNO3
A)1
B)2
C)3
D)4
E)5
In this reaction,what is the correct coefficient for sodium chloride? Pb(NO3)2 + ? NaCl → PbCl2 + ? NaNO3
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
25
How many moles of K2SO4 are in 15.0 g of K2SO4 ?
A)0.172 moles
B)2.61 × 103 moles
C)0.111 moles
D)0.0861 moles
E)0.119 moles
A)0.172 moles
B)2.61 × 103 moles
C)0.111 moles
D)0.0861 moles
E)0.119 moles

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
26
What is the classification for this unbalanced reaction? Fe + HCl → FeCl3 + H2
A)combustion
B)combination
C)decomposition
D)single replacement
E)double replacement
A)combustion
B)combination
C)decomposition
D)single replacement
E)double replacement

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
27
3.00 moles of NO2 have a mass of ________.
A)138 g
B)46.0 g
C)30.0 g
D)90.0 g
E)45.0 g
A)138 g
B)46.0 g
C)30.0 g
D)90.0 g
E)45.0 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
28
Pentane ( C5H12 )reacts with oxygen ( O2 )to form carbon dioxide ( CO2 )and water ( H2O)according to the following reaction.Answer the following question(s)about this reaction. C5H12 + ?O2 → ?CO2 + ?H2O
What is the coefficient for carbon dioxide in the balanced equation?
A)2
B)4
C)5
D)6
E)8
What is the coefficient for carbon dioxide in the balanced equation?
A)2
B)4
C)5
D)6
E)8

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following gives the balanced equation for this reaction? K3PO4 + Ca(NO3)2 → Ca3(PO4)2 + KNO3
A) KPO4+ CaNO3+ KNO3
B) K3PO4 + Ca(NO3)2 → Ca3(PO4)2 + 3KNO3
C)2 K3PO4 + Ca(NO3)2 → Ca3(PO4)2 + 6KNO3
D)2k3PO4+ 3Ca3(NO3)2 → Ca3(PO4)2+ 6KNO3
E) K3PO4+ Ca(NO3)2 → Ca3(PO4)2+ KNO3
A) KPO4+ CaNO3+ KNO3
B) K3PO4 + Ca(NO3)2 → Ca3(PO4)2 + 3KNO3
C)2 K3PO4 + Ca(NO3)2 → Ca3(PO4)2 + 6KNO3
D)2k3PO4+ 3Ca3(NO3)2 → Ca3(PO4)2+ 6KNO3
E) K3PO4+ Ca(NO3)2 → Ca3(PO4)2+ KNO3

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
30
How many grams of K2SO4 are there in 0.123 mole of K2SO4?
A)14.7 g
B)16.6 g
C)21.4 g
D)42.8 g
E)76.3 g
A)14.7 g
B)16.6 g
C)21.4 g
D)42.8 g
E)76.3 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
31
4.00 moles of sodium have a mass of ________.
A)4.60 g
B)11.0 g
C)23.0 g
D)44.0 g
E)92.0 g
A)4.60 g
B)11.0 g
C)23.0 g
D)44.0 g
E)92.0 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
32
A chemical equation is balanced when ________.
A)the total number of molecules is the same in reactants and products
B)the total number of ions is the same in reactants and products
C)the sum of the coefficients of the reactants is equal to the sum of the coefficients of the products
D)the number of atoms of each element is the same in reactants and products
E)the charge on each atom is the same in reactants and products
A)the total number of molecules is the same in reactants and products
B)the total number of ions is the same in reactants and products
C)the sum of the coefficients of the reactants is equal to the sum of the coefficients of the products
D)the number of atoms of each element is the same in reactants and products
E)the charge on each atom is the same in reactants and products

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
33
How many grams of glucose ( C6H12O6 )are in 3.55 moles of glucose?
A)180.g
B)640.g
C)103 g
D)426 g
E)50.7 g
A)180.g
B)640.g
C)103 g
D)426 g
E)50.7 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following correctly gives the correct coefficients for the reaction below? N2H4+ H2O2 → N2 + H2O
A)1,2,1,1
B)1,2,1,4
C)2,4,2,8
D)1,4,1,4
E)2,4,2,4
A)1,2,1,1
B)1,2,1,4
C)2,4,2,8
D)1,4,1,4
E)2,4,2,4

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
35
Pentane ( C5H12 )reacts with oxygen ( O2 )to form carbon dioxide ( CO2 )and water ( H2O)according to the following reaction.Answer the following question(s)about this reaction. C5H12 + ?O2 → ?CO2 + ?H2O
For the following reaction,what is the correct coefficient for the H2? Fe + HCl → FeCl3 + H2
A)1
B)2
C)3
D)4
E)5
For the following reaction,what is the correct coefficient for the H2? Fe + HCl → FeCl3 + H2
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
36
What coefficient is placed in front of O2 to complete the balancing of the following equation? C5H8 + ? O2 → 5CO2+ 4H2O
A)1
B)3
C)5
D)7
E)9
A)1
B)3
C)5
D)7
E)9

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
37
In any balanced chemical equation,the number of each type of atom on both sides of the equation is ________.
A)doubled
B)the same
C)decreased by one
D)increased by one
E)dependent on the temperature
A)doubled
B)the same
C)decreased by one
D)increased by one
E)dependent on the temperature

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
38
Pentane ( C5H12 )reacts with oxygen ( O2 )to form carbon dioxide ( CO2 )and water ( H2O)according to the following reaction.Answer the following question(s)about this reaction. C5H12 + ?O2 → ?CO2 + ?H2O
What is the coefficient for oxygen in the balanced equation?
A)2
B)4
C)5
D)6
E)8
What is the coefficient for oxygen in the balanced equation?
A)2
B)4
C)5
D)6
E)8

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
39
Pentane ( C5H12 )reacts with oxygen ( O2 )to form carbon dioxide ( CO2 )and water ( H2O)according to the following reaction.Answer the following question(s)about this reaction. C5H12 + ?O2 → ?CO2 + ?H2O
What is the coefficient for water in the balanced equation?
A)2
B)4
C)5
D)6
E)8
What is the coefficient for water in the balanced equation?
A)2
B)4
C)5
D)6
E)8

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
40
How many grams of Fe2O3 are there in 0.500 mole of Fe2O3 ?
A)79.9 g
B)35.9 g
C)63.8 g
D)51.9 g
E)160.g
A)79.9 g
B)35.9 g
C)63.8 g
D)51.9 g
E)160.g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
41
In an oxidation-reduction reaction,the substance oxidized always ________.
A)takes on oxygen atoms
B)loses electrons
C)gives up hydrogen atoms
D)gains electrons
E)becomes a charged species
A)takes on oxygen atoms
B)loses electrons
C)gives up hydrogen atoms
D)gains electrons
E)becomes a charged species

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
42
For the following question(s),consider the following balanced equation. Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH3
In the reaction of nitrogen gas, N2 ,with hydrogen gas, H2 ,to form ammonia gas,NH3,how many moles of hydrogen are needed to react with two moles of nitrogen? N2 + 3H2 → 2NH3
A)2 moles
B)4 moles
C)6 moles
D)8 moles
E)5.710 moles
In the reaction of nitrogen gas, N2 ,with hydrogen gas, H2 ,to form ammonia gas,NH3,how many moles of hydrogen are needed to react with two moles of nitrogen? N2 + 3H2 → 2NH3
A)2 moles
B)4 moles
C)6 moles
D)8 moles
E)5.710 moles

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
43
For the following question(s),consider the following equation.
2Mg + O2 → 2MgO
How many grams of magnesium are needed to react with 16 g of O2 ?
A)0.50 g
B)1.0 g
C)12 g
D)24 g
E)48 g
2Mg + O2 → 2MgO
How many grams of magnesium are needed to react with 16 g of O2 ?
A)0.50 g
B)1.0 g
C)12 g
D)24 g
E)48 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
44
In a ________ reaction,two or more elements or compounds form one product.
A)decomposition
B)single replacement
C)combustion
D)double replacement
E)combination
A)decomposition
B)single replacement
C)combustion
D)double replacement
E)combination

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
45
The following reaction takes place when an electric current is passed through water.It is an example of a ________ reaction. 2H2O 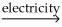 2H2 + O2
2H2 + O2
A)combination
B)single replacement
C)combustion
D)decomposition
E)double replacement
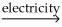 2H2 + O2
2H2 + O2A)combination
B)single replacement
C)combustion
D)decomposition
E)double replacement

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
46
For the following question(s),consider the following balanced equation. Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH3
When 2 moles of Mg3N2 are allowed to react,how many moles of H2O also react?
A)1 mole
B)4 moles
C)6 moles
D)8 moles
E)12 moles
When 2 moles of Mg3N2 are allowed to react,how many moles of H2O also react?
A)1 mole
B)4 moles
C)6 moles
D)8 moles
E)12 moles

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
47
For the following question(s),consider the following equation.
2Mg + O2 → 2MgO
How many grams of MgO are produced when 40.0 grams of O2 react completely with Mg?
A)40.3 g
B)50.4 g
C)60.5 g
D)101 g
E)202 g
2Mg + O2 → 2MgO
How many grams of MgO are produced when 40.0 grams of O2 react completely with Mg?
A)40.3 g
B)50.4 g
C)60.5 g
D)101 g
E)202 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
48
For the following question(s),consider the following balanced equation. Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH3
What is the correct form of the conversion factor needed to convert the number of moles of H2O to the number of moles of NH3 produced?
A)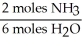
B)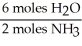
C)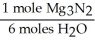
D)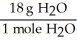
E)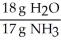
What is the correct form of the conversion factor needed to convert the number of moles of H2O to the number of moles of NH3 produced?
A)
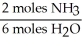
B)
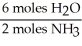
C)
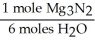
D)
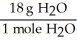
E)
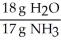

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
49
For the following question(s),consider the following balanced equation. Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH3
When 4 moles of aluminum are allowed to react with an excess of chlorine gas, Cl2 ,how many moles of aluminum chloride are produced? 2Al + 3Cl2 → 2AlCl3
A)1 mole
B)2 moles
C)3 moles
D)4 moles
E)5 moles
When 4 moles of aluminum are allowed to react with an excess of chlorine gas, Cl2 ,how many moles of aluminum chloride are produced? 2Al + 3Cl2 → 2AlCl3
A)1 mole
B)2 moles
C)3 moles
D)4 moles
E)5 moles

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
50
What is the classification for this reaction? SO3 + H2O → H2SO4
A)decomposition
B)combination
C)replacement
D)double replacement
E)combustion
A)decomposition
B)combination
C)replacement
D)double replacement
E)combustion

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
51
For the following question(s),consider the following equation.
2Mg + O2 → 2MgO
The number of moles of MgO produced when 0.20 mole of O2 reacts completely is ________.
A)0.10 mole
B)0.20 mole
C)0.40 mole
D)0.60 mole
E)0.80 mole
2Mg + O2 → 2MgO
The number of moles of MgO produced when 0.20 mole of O2 reacts completely is ________.
A)0.10 mole
B)0.20 mole
C)0.40 mole
D)0.60 mole
E)0.80 mole

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
52
The following reaction is an example of a ________ reaction. C5H8 + 7O2 → 5CO2 + 4H2O
A)combination
B)single replacement
C)combustion
D)decomposition
E)double replacement
A)combination
B)single replacement
C)combustion
D)decomposition
E)double replacement

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
53
For the following question(s),consider the following equation.
2Mg + O2 → 2MgO
The number of moles of oxygen gas needed to react with 4.0 moles of Mg is ________.
A)1.0 mole
B)2.0 moles
C)3.0 moles
D)4.0 moles
E)6.0 moles
2Mg + O2 → 2MgO
The number of moles of oxygen gas needed to react with 4.0 moles of Mg is ________.
A)1.0 mole
B)2.0 moles
C)3.0 moles
D)4.0 moles
E)6.0 moles

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
54
In this reaction,what is the substance oxidized? Zn + 2 HCl → ZnCl2 + H2
A)chlorine
B)zinc chloride
C)hydrogen
D)zinc
E)oxygen
A)chlorine
B)zinc chloride
C)hydrogen
D)zinc
E)oxygen

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
55
Given the following equation,what is the correct form of the conversion factor needed to convert the number of moles of O2 to the number of moles of Fe2O3 produced? 4Fe + 3O2 → 2Fe2O3
A)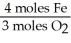
B)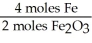
C)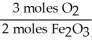
D)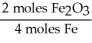
E)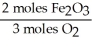
A)
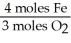
B)
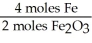
C)
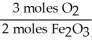
D)
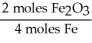
E)
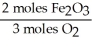

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is an oxidation-reduction reaction?
A)CaCl2 + Na2SO4 → CaSO4 + 2NaCl
B)KOH + HNO3 → H2O + KNO3
C) N2 + O2 → 2NO
D) AgNO3 + NaCl → AgCl + NaNO3
E) Al2(SO4)3 + 6KOH → 2Al(OH)3 + 3K2SO4
A)CaCl2 + Na2SO4 → CaSO4 + 2NaCl
B)KOH + HNO3 → H2O + KNO3
C) N2 + O2 → 2NO
D) AgNO3 + NaCl → AgCl + NaNO3
E) Al2(SO4)3 + 6KOH → 2Al(OH)3 + 3K2SO4

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
57
In an oxidation-reduction reaction,the substance reduced always ________.
A)takes on oxygen atoms
B)loses electrons
C)gives up hydrogen atoms
D)gains electrons
E)becomes a charged species
A)takes on oxygen atoms
B)loses electrons
C)gives up hydrogen atoms
D)gains electrons
E)becomes a charged species

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
58
The reaction of carbon with oxygen to produce carbon monoxide is an example of which class of reaction? 2C + O2 → 2CO
A)single replacement
B)double replacement
C)combination
D)catalytic
E)endothermic
A)single replacement
B)double replacement
C)combination
D)catalytic
E)endothermic

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
59
For the following question(s),consider the following equation.
2Mg + O2 → 2MgO
How many grams of hydrogen are needed to produce 1.80 g of water according to this equation? 2H2 + O2 → 2H2O
A)0.101 g
B)0.180 g
C)0.201 g
D)2.01 g
E)4.02 g
2Mg + O2 → 2MgO
How many grams of hydrogen are needed to produce 1.80 g of water according to this equation? 2H2 + O2 → 2H2O
A)0.101 g
B)0.180 g
C)0.201 g
D)2.01 g
E)4.02 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
60
What is oxidized and what is reduced in the following reaction? 2Al + 3Br2 → 2AlBr3
A)Al is oxidized and Br2 is reduced.
B)AlBr3 is reduced and Br2 is oxidized.
C)Al is reduced and Br2 is oxidized.
D) AlBr3 is reduced and Al is oxidized.
E) AlBr3 is oxidized and Al is reduced.
A)Al is oxidized and Br2 is reduced.
B)AlBr3 is reduced and Br2 is oxidized.
C)Al is reduced and Br2 is oxidized.
D) AlBr3 is reduced and Al is oxidized.
E) AlBr3 is oxidized and Al is reduced.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
61
Barium chloride and sodium sulfate react according to the following equation.
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Answer the following question(s)about this reaction.
How many grams of sodium chloride can be produced from 50.grams of barium chloride?
A)2.0 g
B)14 g
C)28 g
D)59 g
E)208 g
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Answer the following question(s)about this reaction.
How many grams of sodium chloride can be produced from 50.grams of barium chloride?
A)2.0 g
B)14 g
C)28 g
D)59 g
E)208 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
62
For the following question(s),consider the following balanced equation. Mg3N2+ 6H2O → 3Mg(OH)2 + 2NH3
How many grams of H2O are needed to produce 150 g of Mg(OH)2 ?
A)46 g
B)18 g
C)130 g
D)93 g
E)23 g
How many grams of H2O are needed to produce 150 g of Mg(OH)2 ?
A)46 g
B)18 g
C)130 g
D)93 g
E)23 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
63
For the following question(s),consider the following balanced equation. Mg3N2+ 6H2O → 3Mg(OH)2 + 2NH3
How many grams of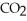 are produced from 125 g of
are produced from 125 g of 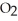 and excess
and excess 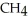 ?
?  + 2
+ 2 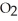 →
→ 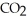 + 2
+ 2 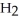 O
O
A)125 g
B)62.5 g
C)172 g
D)85.9 g
E)250.g
How many grams of
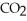 are produced from 125 g of
are produced from 125 g of 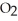 and excess
and excess 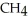 ?
?  + 2
+ 2 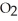 →
→ 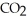 + 2
+ 2 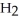 O
OA)125 g
B)62.5 g
C)172 g
D)85.9 g
E)250.g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
64
If the reaction shown below is exothermic,the energy level of the reactants is ________. 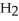 +
+ 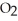 →
→ 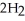 O
O
A)lower than that of the products
B)higher than that of the products
C)the same as that of the products
D)possibly lower,possibly higher than that of the products
E)higher than the activation energy of the reaction
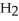 +
+ 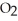 →
→ 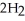 O
OA)lower than that of the products
B)higher than that of the products
C)the same as that of the products
D)possibly lower,possibly higher than that of the products
E)higher than the activation energy of the reaction

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
65
Answer the question(s)below about the following reaction.
2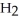
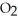 → 2
→ 2 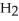 O +
O + 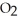
How many moles of oxygen gas can 5.0 moles of hydrogen peroxide ( H2O2)produce,if decomposition is complete?
A)5.0 moles
B)2.5 moles
C)1.0 mole
D)2.0 moles
E)10.moles
2
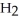
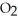 → 2
→ 2 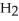 O +
O + 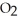
How many moles of oxygen gas can 5.0 moles of hydrogen peroxide ( H2O2)produce,if decomposition is complete?
A)5.0 moles
B)2.5 moles
C)1.0 mole
D)2.0 moles
E)10.moles

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
66
For the following question(s),consider the following balanced equation. Mg3N2+ 6H2O → 3Mg(OH)2 + 2NH3
How many grams of NO are required to produce 145 g of N2 in the following reaction? 4NH3 + 6NO → 5N2 + 6H2O
A)186 g
B)155 g
C)125 g
D)129 g
E)145 g
How many grams of NO are required to produce 145 g of N2 in the following reaction? 4NH3 + 6NO → 5N2 + 6H2O
A)186 g
B)155 g
C)125 g
D)129 g
E)145 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
67
The ________ is the minimum energy needed for a chemical reaction to begin.
A)reaction energy
B)activation energy
C)energy of reactants
D)energy of products
E)heat of reaction
A)reaction energy
B)activation energy
C)energy of reactants
D)energy of products
E)heat of reaction

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
68
A reaction that releases energy as it occurs is classified as a(n)________.
A)endothermic reaction
B)exothermic reaction
C)oxidation-reduction reaction
D)catalyzed reaction
E)decomposition reaction
A)endothermic reaction
B)exothermic reaction
C)oxidation-reduction reaction
D)catalyzed reaction
E)decomposition reaction

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
69
For the following question(s),consider the following balanced equation. Mg3N2+ 6H2O → 3Mg(OH)2 + 2NH3
When 36.0 g of H2O react,how many grams of NH3 are produced?
A)34.0 g
B)10.0 g
C)5.67 g
D)11.3 g
E)102 g
When 36.0 g of H2O react,how many grams of NH3 are produced?
A)34.0 g
B)10.0 g
C)5.67 g
D)11.3 g
E)102 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
70
Barium chloride and sodium sulfate react according to the following equation.
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Answer the following question(s)about this reaction.
How many moles of barium sulfate are produced from 0.1 mole of barium chloride?
A)0.01 mole
B)0.1 mole
C)0.2 mole
D)1 mole
E)2 moles
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Answer the following question(s)about this reaction.
How many moles of barium sulfate are produced from 0.1 mole of barium chloride?
A)0.01 mole
B)0.1 mole
C)0.2 mole
D)1 mole
E)2 moles

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
71
Any reaction that absorbs 150 kcal of energy can be classified as ________.
A)endothermic
B)exothermic
C)activated
D)reduction
E)oxidation
A)endothermic
B)exothermic
C)activated
D)reduction
E)oxidation

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
72
For the following question(s),consider the following equation.
2Mg + O2 → 2MgO
In the reaction of silver nitrate with sodium chloride,how many grams of silver chloride will be produced from 100.g of silver nitrate when it is mixed with an excess of sodium chloride? The equation for the reaction is below. AgNO3 + NaCl → AgCl + NaNO3
A)107.9 g
B)169.9 g
C)84.4 g
D)0.589 g
E)58.9 g
2Mg + O2 → 2MgO
In the reaction of silver nitrate with sodium chloride,how many grams of silver chloride will be produced from 100.g of silver nitrate when it is mixed with an excess of sodium chloride? The equation for the reaction is below. AgNO3 + NaCl → AgCl + NaNO3
A)107.9 g
B)169.9 g
C)84.4 g
D)0.589 g
E)58.9 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
73
In this reaction,what is the correct coefficient for hydrogen gas? ? H2 + ? O2 → ? H2O
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
74
Barium chloride and sodium sulfate react according to the following equation.
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Answer the following question(s)about this reaction.
How many grams of barium chloride are needed to make 100.grams of barium sulfate?
A)44.9 g
B)89.2 g
C)208 g
D)233 g
E)46.6 g
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Answer the following question(s)about this reaction.
How many grams of barium chloride are needed to make 100.grams of barium sulfate?
A)44.9 g
B)89.2 g
C)208 g
D)233 g
E)46.6 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
75
Answer the question(s)below about the following reaction.
2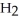
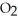 → 2
→ 2 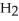 O +
O + 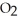
How many grams of water will 100.grams of hydrogen peroxide produce?
A)36.0 g
B)360.g
C)5.88 g
D)53.0 g
E)106 g
2
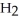
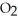 → 2
→ 2 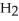 O +
O + 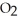
How many grams of water will 100.grams of hydrogen peroxide produce?
A)36.0 g
B)360.g
C)5.88 g
D)53.0 g
E)106 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
76
What type of reaction is: 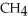 + 2
+ 2 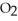 →
→ 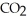 + 2
+ 2 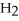 O + 218 kcal?
O + 218 kcal?
A)an endothermic reaction
B)an exothermic reaction
C)a single replacement reaction
D)a combination reaction
E)a decomposition reaction
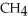 + 2
+ 2 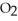 →
→ 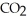 + 2
+ 2 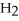 O + 218 kcal?
O + 218 kcal?A)an endothermic reaction
B)an exothermic reaction
C)a single replacement reaction
D)a combination reaction
E)a decomposition reaction

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
77
For the following question(s),consider the following balanced equation. Mg3N2+ 6H2O → 3Mg(OH)2 + 2NH3
How many grams of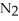 are produced when 125 g of
are produced when 125 g of 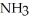 reacts as shown in the following balanced equation? 4
reacts as shown in the following balanced equation? 4 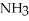 + 6NO → 5
+ 6NO → 5 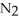 + 6
+ 6 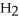 O
O
A)206 g
B)257 g
C)165 g
D)7.35 g
E)145 g
How many grams of
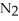 are produced when 125 g of
are produced when 125 g of 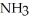 reacts as shown in the following balanced equation? 4
reacts as shown in the following balanced equation? 4 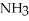 + 6NO → 5
+ 6NO → 5 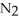 + 6
+ 6 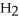 O
OA)206 g
B)257 g
C)165 g
D)7.35 g
E)145 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
78
Answer the question(s)below about the following reaction.
2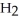
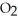 → 2
→ 2 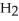 O +
O + 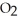
This is an example of a ________ reaction.
A)combustion
B)combination
C)decomposition
D)single replacement
E)double replacement
2
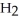
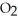 → 2
→ 2 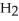 O +
O + 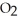
This is an example of a ________ reaction.
A)combustion
B)combination
C)decomposition
D)single replacement
E)double replacement

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
79
The ________ is the energy difference between reactants and products in a chemical reaction.
A)transition energy
B)activation energy
C)product energy
D)overall energy
E)heat of reaction
A)transition energy
B)activation energy
C)product energy
D)overall energy
E)heat of reaction

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
80
For the following question(s),consider the following balanced equation. Mg3N2+ 6H2O → 3Mg(OH)2 + 2NH3
Find the mass of AlCl3 that is produced when 25.0 grams of Al2O3 reacts with HCl according to the following equation. Al2O3 + 6HCl → 2AlCl3 + 3H2O
A)155 g
B)72.9 g
C)65.3 g
D)32.6 g
E)16.3 g
Find the mass of AlCl3 that is produced when 25.0 grams of Al2O3 reacts with HCl according to the following equation. Al2O3 + 6HCl → 2AlCl3 + 3H2O
A)155 g
B)72.9 g
C)65.3 g
D)32.6 g
E)16.3 g

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck



