Deck 10: Thermal Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/84
Play
Full screen (f)
Deck 10: Thermal Physics
1
A steel wire,150 m long at 10 C,has a coefficient of linear expansion of 11 x 10-6/C .Give its change in length as the temperature changes from 10 C to 45 C.
A)0.65 cm
B)1.8 cm
C)5.8 cm
D)12 cm
A)0.65 cm
B)1.8 cm
C)5.8 cm
D)12 cm
5.8 cm
2
If it is given that 546 K equals 273 C,then it follows that 400 K equals:
A)127 C.
B)150 C.
C)473 C.
D)1 200 C.
A)127 C.
B)150 C.
C)473 C.
D)1 200 C.
127 C.
3
At what temperature is the same numerical value obtained in Celsius and Fahrenheit?
A)-40
B)0
C)40
D)-72
A)-40
B)0
C)40
D)-72
-40
4
The observation that materials expand in size with an increase in temperature can be applied to what proportion of existing substances?
A)100%
B)most
C)few
D)none
A)100%
B)most
C)few
D)none

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following properties can be used to measure temperature?
A)the color of a glowing object
B)the length of a solid
C)the volume of gas held at constant pressure
D)all of the above
A)the color of a glowing object
B)the length of a solid
C)the volume of gas held at constant pressure
D)all of the above

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
6
Carbon dioxide forms into a solid (dry ice)at approximately -157 F.What temperature in degrees Celsius does this correspond to?
A)-157 C
B)-93 C
C)-121 C
D)-105 C
A)-157 C
B)-93 C
C)-121 C
D)-105 C

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
7
88 F is how many degrees Celsius?
A)31
B)49
C)56
D)158
A)31
B)49
C)56
D)158

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
8
Normal body temperature for humans is 37 C.What is this temperature in kelvins?
A)296
B)310
C)393
D)273
A)296
B)310
C)393
D)273

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
9
The pressure in a constant-volume gas thermometer extrapolates to zero at what temperature?
A)0 C
B)0 K
C)0 F
D)0 Pa
A)0 C
B)0 K
C)0 F
D)0 Pa

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
10
A substance is heated from 15 C to 35 C.What would the same incremental change be when registered in kelvins?
A)20
B)40
C)36
D)313
A)20
B)40
C)36
D)313

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
11
Which best describes a system made up of ice,water and steam existing together?
A)absolute zero
B)triple point
C)ice point
D)steam point
A)absolute zero
B)triple point
C)ice point
D)steam point

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
12
A rectangular steel plate with dimensions of 30 cm x 25 cm is heated from 20 C to 220 C.What is its change in area? (Coefficient of linear expansion for steel is 11 x 10-6/C . )
A)0.82 cm2
B)1.65 cm2
C)3.3 cm2
D)6.6 cm2
A)0.82 cm2
B)1.65 cm2
C)3.3 cm2
D)6.6 cm2

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
13
The zeroth law of thermodynamics pertains to what relational condition that may exist between two systems?
A)zero net forces
B)zero velocities
C)zero temperature
D)thermal equilibrium
A)zero net forces
B)zero velocities
C)zero temperature
D)thermal equilibrium

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
14
Which best describes the relationship between two systems in thermal equilibrium?
A)no net energy is exchanged
B)volumes are equal
C)masses are equal
D)zero velocity
A)no net energy is exchanged
B)volumes are equal
C)masses are equal
D)zero velocity

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
15
What is the temperature of a system in thermal equilibrium with another system made up of ice and water at one atmosphere of pressure?
A)0 F
B)273 K
C)0 K
D)100 C
A)0 F
B)273 K
C)0 K
D)100 C

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
16
An interval of one Celsius degree is equivalent to an interval of:
A)one Fahrenheit degree.
B)one kelvin.
C)5/9 Fahrenheit degree.
D)5/9 kelvin.
A)one Fahrenheit degree.
B)one kelvin.
C)5/9 Fahrenheit degree.
D)5/9 kelvin.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
17
A temperature change from 15 C to 35 C corresponds to what incremental change in F?
A)20
B)40
C)36
D)313
A)20
B)40
C)36
D)313

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
18
What happens to a given mass of water as it is cooled from 4 C to zero?
A)expands
B)contracts
C)vaporizes
D)Neither expands,contracts,nor vaporizes.
A)expands
B)contracts
C)vaporizes
D)Neither expands,contracts,nor vaporizes.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
19
What is the temperature of a system in thermal equilibrium with another system made up of water and steam at one atmosphere of pressure?
A)0 F
B)273 K
C)0 K
D)100 C
A)0 F
B)273 K
C)0 K
D)100 C

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
20
A temperature of 233 K equals which of the following?
A)506 C
B)40 C
C)-40 F
D)40 F
A)506 C
B)40 C
C)-40 F
D)40 F

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
21
An ideal gas is confined to a container with adjustable volume.The pressure and mole number are constant.By what factor will volume change if absolute temperature triples?
A)1/9
B)1/3
C)3.0
D)9.0
A)1/9
B)1/3
C)3.0
D)9.0

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
22
A material has a coefficient of volume expansion of 60 x 10-6/ C.What is its area coefficient of expansion?
A)120 x 10-6/ C
B)40 x 10-6/ C
C)20 x 10-6/ C
D)180 x 10-6/ C
A)120 x 10-6/ C
B)40 x 10-6/ C
C)20 x 10-6/ C
D)180 x 10-6/ C

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
23
Suppose the ends of a 20-m-long steel beam are rigidly clamped at 0 C to prevent expansion.The rail has a cross-sectional area of 30 cm2.What force does the beam exert when it is heated to 40 C? (asteel = 1.1 x 10-5/C ,Ysteel = 2.0 x 1011 N/m2).
A)2.6 x 105 N
B)5.6 x 104 N
C)1.3 x 103 N
D)6.5 x 102 N
A)2.6 x 105 N
B)5.6 x 104 N
C)1.3 x 103 N
D)6.5 x 102 N

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
24
The coefficient of area expansion is:
A)half the coefficient of volume expansion.
B)three halves the coefficient of volume expansion.
C)double the coefficient of linear expansion.
D)triple the coefficient of linear expansion.
A)half the coefficient of volume expansion.
B)three halves the coefficient of volume expansion.
C)double the coefficient of linear expansion.
D)triple the coefficient of linear expansion.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
25
An automobile gas tank is filled to its capacity of 15.00 gallons with the gasoline at an initial temperature of 10 C.The automobile is parked in the sun causing the gasoline's temperature to rise to 60 C.If the coefficient of volume expansion for gasoline is 9.6 x 10-4/C ,what volume runs out the overflow tube? Assume the change in volume of the tank is negligible.
A)1.74 gallons
B)1.18 gallons
C)0.72 gallons
D)0.30 gallons
A)1.74 gallons
B)1.18 gallons
C)0.72 gallons
D)0.30 gallons

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
26
The thermal expansion of a solid is caused by:
A)the breaking of bonds between atoms.
B)increasing the amplitude of the atoms vibration.
C)increasing the distance between equilibrium positions for the vibrating atoms.
D)all of the above.
A)the breaking of bonds between atoms.
B)increasing the amplitude of the atoms vibration.
C)increasing the distance between equilibrium positions for the vibrating atoms.
D)all of the above.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
27
A steel sphere sits on top of an aluminum ring.The steel sphere (a = 1.10 x 10-5/C )has a diameter of 4.000 0 cm at 0 C.The aluminum ring (a = 2.40 x 10-5/C )has an inside diameter of 3.994 0 cm at 0 C.Closest to which temperature given will the sphere just fall through the ring?
A)462 C
B)208 C
C)116 C
D)57.7 C
A)462 C
B)208 C
C)116 C
D)57.7 C

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
28
What happens to a volume of water when its temperature is reduced from 8 C to 4 C?
A)density increases
B)density decreases
C)density remains constant
D)vaporizes
A)density increases
B)density decreases
C)density remains constant
D)vaporizes

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
29
At 20 C an aluminum ring has an inner diameter of 5.000 cm,and a brass rod has a diameter of 5.050 cm.Keeping the brass rod at 20 C,which of the following temperatures of the ring will allow the ring to just slip over the brass rod? (aAl = 2.4 x 10-5 /C ,abrass = 1.9 x 10-5/C )
A)111 C
B)236 C
C)384 C
D)437 C
A)111 C
B)236 C
C)384 C
D)437 C

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
30
At room temperature,the coefficient of linear expansion for Pyrex glass is ____ that for ordinary glass.
A)the same as
B)more than
C)less than
D)stronger than
A)the same as
B)more than
C)less than
D)stronger than

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
31
A pipe of length 10.0 m increases in length by 1.5 cm when its temperature is increased by 90 F.What is its coefficient of linear expansion?
A)30 x 10-6/ C
B)17 x 10-6/ C
C)13 x 10-6/ C
D)23 x 10-6/ C
A)30 x 10-6/ C
B)17 x 10-6/ C
C)13 x 10-6/ C
D)23 x 10-6/ C

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
32
What happens to a given volume of water when heated from 0 C to 4 C?
A)density increases
B)density decreases
C)density remains constant
D)vaporizes
A)density increases
B)density decreases
C)density remains constant
D)vaporizes

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
33
A steel plate has a hole drilled through it.The plate is put into a furnace and heated.What happens to the size of the inside diameter of a hole as its temperature increases?
A)increases
B)decreases
C)remains constant
D)becomes elliptical
A)increases
B)decreases
C)remains constant
D)becomes elliptical

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
34
A brass cube,10 cm on a side,is raised in temperature by 200 C.The coefficient of volume expansion of brass is 57 x 10-6/C .By what percentage does volume increase?
A)12%
B)2.8%
C)1.1%
D)0.86%
A)12%
B)2.8%
C)1.1%
D)0.86%

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
35
Between 0 and 4 C,the volume coefficient of expansion for water:
A)is positive.
B)is zero.
C)is becoming less dense.
D)is negative.
A)is positive.
B)is zero.
C)is becoming less dense.
D)is negative.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
36
What happens to its moment of inertia when a steel disk is heated?
A)It increases.
B)It decreases.
C)It stays the same.
D)It increases for half the temperature increase and then decreases for the rest of the temperature increase.
A)It increases.
B)It decreases.
C)It stays the same.
D)It increases for half the temperature increase and then decreases for the rest of the temperature increase.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
37
A long steel beam has a length of twenty-five meters on a cold day when the temperature is 0 C.What is the length of the beam on a hot day when T = 40 C? (asteel = 1.1 x 10-5/C )
A)25.000 44 m
B)25.004 4 m
C)25.011 m
D)25.044 m
A)25.000 44 m
B)25.004 4 m
C)25.011 m
D)25.044 m

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
38
Which best expresses the value for the coefficient of volume expansion,b,for given material as a function of its corresponding coefficient of linear expansion,a?
A)b = a 3
B)b = 3a
C)b = a 2
D)b = 2a
A)b = a 3
B)b = 3a
C)b = a 2
D)b = 2a

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
39
As a copper wire is heated,its length increases by 0.100%.What is the change of the temperature of the wire? (aCu = 16.6 x 10-6/C )
A)120.4 C
B)60.2 C
C)30.1 C
D)6.0 C
A)120.4 C
B)60.2 C
C)30.1 C
D)6.0 C

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
40
A brass cube,10 cm on a side,is raised in temperature by 200 C.The coefficient of volume expansion of brass is 57 x 10-6/C .By what percentage is any one of the 10-cm edges increased in length?
A)4%
B)2.8%
C)0.38%
D)0.29%
A)4%
B)2.8%
C)0.38%
D)0.29%

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
41
9.0 g of water in a 2.0-L pressure vessel is heated to 500 C.What is the pressure inside the container? (R = 0.082 L*atm/mol*K,one mole of water has a mass of 18 grams)
A)7.9 atm
B)16 atm
C)24 atm
D)32 atm
A)7.9 atm
B)16 atm
C)24 atm
D)32 atm

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
42
A pressure of 1.0 x 10-7 mm of Hg is achieved in a vacuum system.How many gas molecules are present per liter volume if the temperature is 293 K? (760 mm of Hg = 1 atm,R = 0.082 1 L*atm/mol*K,and NA = 6.02 x 1023)
A)16 x 1018
B)4.7 x 1016
C)3.3 x 1012
D)3.4 x 109
A)16 x 1018
B)4.7 x 1016
C)3.3 x 1012
D)3.4 x 109

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
43
Tricia puts 44 g of dry ice (solid CO2)into a 2.0-L container and seals the top.The dry ice turns to gas at room temperature (20 C).Find the pressure increase in the 2.0-L container.(One mole of CO2 has a mass of 44 g,R = 0.082 1 L*atm/mol*K.Ignore the initial volume of the dry ice. )
A)6.0 atm
B)12 atm
C)18 atm
D)2.0 atm
A)6.0 atm
B)12 atm
C)18 atm
D)2.0 atm

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
44
Two moles of nitrogen gas are contained in an enclosed cylinder with a movable piston.If the gas temperature is 298 K,and the pressure is 1.01 x 106 N/m2,what is the volume? (R = 8.31 J/mol*K)
A)9.80 x 10-3 m3
B)4.90 x 10-3 m3
C)17.3 x 10-3 m3
D)8.31 x 10-3 m3
A)9.80 x 10-3 m3
B)4.90 x 10-3 m3
C)17.3 x 10-3 m3
D)8.31 x 10-3 m3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
45
With molar quantity and temperature held constant,by what factor does the pressure of an ideal gas change when the volume is five times bigger?
A)0.2
B)1.0
C)5.0
D)25.0
A)0.2
B)1.0
C)5.0
D)25.0

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
46
With volume and molar quantity held constant,by what factor does the absolute temperature change for an ideal gas when the pressure is five times bigger?
A)0.2
B)1.0
C)5.0
D)25.0
A)0.2
B)1.0
C)5.0
D)25.0

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
47
Estimate the volume of a helium-filled balloon at STP if it is to lift a payload of 500 kg.The density of air is 1.29 kg/m3 and helium has a density of 0.178 kg/m3.
A)4 410 m3
B)932 m3
C)450 m3
D)225 m3
A)4 410 m3
B)932 m3
C)450 m3
D)225 m3

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
48
An ideal gas is confined to a container with constant volume.The number of moles is constant.By what factor will the pressure change if the absolute temperature triples?
A)1/9
B)1/3
C)3.0
D)9.0
A)1/9
B)1/3
C)3.0
D)9.0

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
49
How many atoms are present in a sample of pure iron with a mass of 300 g? (The atomic mass of iron = 56 and NA = 6.02 x 1023)
A)1.8 x 1019
B)6.7 x 1022
C)1.6 x 1028
D)3.2 x 1024
A)1.8 x 1019
B)6.7 x 1022
C)1.6 x 1028
D)3.2 x 1024

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
50
Two moles of nitrogen gas are contained in an enclosed cylinder with a movable piston.If the molecular mass of nitrogen is 28,how many grams of nitrogen are present?
A)0.14
B)56
C)42
D)112
A)0.14
B)56
C)42
D)112

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
51
Boltzmann's constant,kB,may be derived as a function of R,the universal gas constant,and NA,Avogadro's number.Which expresses the value of kB?
A)NAR2
B)NAR
C)R/NA
D)NA/R
A)NAR2
B)NAR
C)R/NA
D)NA/R

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
52
One mole of an ideal gas at 1.00 atm and 0.00 C occupies 22.4 L.How many molecules of an ideal gas are in one cm3 under these conditions?
A)28.9
B)22 400
C)2.69 x 1019
D)6.02 x 1023
A)28.9
B)22 400
C)2.69 x 1019
D)6.02 x 1023

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
53
Two moles of an ideal gas at 3.0 atm and 10 C are heated up to 150 C.If the volume is held constant during this heating,what is the final pressure?
A)4.5 atm
B)1.8 atm
C)0.14 atm
D)1.0 atm
A)4.5 atm
B)1.8 atm
C)0.14 atm
D)1.0 atm

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
54
A 2.00-L container holds half a mole of an ideal gas at a pressure of 12.5 atm.What is the gas temperature? (R = 0.082 1 L*atm/mol*K)
A)1 980 K
B)1 190 K
C)965 K
D)609 K
A)1 980 K
B)1 190 K
C)965 K
D)609 K

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
55
A helium-filled weather balloon has a 0.90 m radius at liftoff where air pressure is 1.0 atm and the temperature is 298 K.When airborne,the temperature is 210 K,and its radius expands to 3.0 m.What is the pressure at the airborne location?
A)0.50 atm
B)0.013 atm
C)0.019 atm
D)0.38 atm
A)0.50 atm
B)0.013 atm
C)0.019 atm
D)0.38 atm

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
56
An ideal gas is confined to a container with adjustable volume.The number of moles and temperature are constant.By what factor will the volume change if pressure triples?
A)1/9
B)1/3
C)3.0
D)9.0
A)1/9
B)1/3
C)3.0
D)9.0

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
57
How many moles of air must escape from a 10-m x 8.0-m x 5.0-m room when the temperature is raised from 0 C to 20 C? Assume the pressure remains unchanged at one atmosphere while the room is heated.
A)1.3 x 103 moles
B)1.2 x 103 moles
C)7.5 x 102 moles
D)3.7 x 102 moles
A)1.3 x 103 moles
B)1.2 x 103 moles
C)7.5 x 102 moles
D)3.7 x 102 moles

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
58
A spherical air bubble originating from a scuba diver at a depth of 18.0 m has a diameter of 1.0 cm.What will the bubble's diameter be when it reaches the surface? (Assume constant temperature. )
A)0.7 cm
B)1.0 cm
C)1.4 cm
D)1.7 cm
A)0.7 cm
B)1.0 cm
C)1.4 cm
D)1.7 cm

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
59
The mass of a hot-air balloon and its cargo (not including the air inside)is 200 kg.The air outside is at a temperature of 10 C and a pressure of 1 atm = 105 N/m2.The volume of the balloon is 400 m3.Which temperature below of the air in the balloon will allow the balloon to just lift off? (Air density at 10 C is 1.25 kg/m3. )
A)37 C
B)69 C
C)99 C
D)200 C
A)37 C
B)69 C
C)99 C
D)200 C

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
60
One way to heat a gas is to compress it.A gas at 1.00 atm at 25.0 C is compressed to one tenth of its original volume,and it reaches 40.0 atm pressure.What is its new temperature?
A)1 500 K
B)1 500 C
C)1 192 C
D)919 C
A)1 500 K
B)1 500 C
C)1 192 C
D)919 C

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
61
The absolute temperature of an ideal gas is directly proportional to which of the following properties,when taken as an average,of the molecules of that gas?
A)speed
B)momentum
C)mass
D)kinetic energy
A)speed
B)momentum
C)mass
D)kinetic energy

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
62
What is the internal energy of 50 moles of Neon gas (molecular mass = 20 u)at 27 C? (R = 8.31 J/mol*K)
A)1.9 x 105 J
B)1.6 x 105 J
C)3.8 x 103 J
D)It depends on the container size,which is not given.
A)1.9 x 105 J
B)1.6 x 105 J
C)3.8 x 103 J
D)It depends on the container size,which is not given.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
63
The internal energy of a monatomic ideal gas is equal to which of the following?
A)(3/2)PV
B)(3/2)nT/V
C)3 T/P
D)none of the above
A)(3/2)PV
B)(3/2)nT/V
C)3 T/P
D)none of the above

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
64
A quantity of a monatomic ideal gas expands to twice the volume while maintaining the same pressure.If the internal energy of the gas were U0 before the expansion,what is it after the expansion?
A)U0
B)2 U0
C)4 U0
D)The change in temperature must also be known to answer this question.
A)U0
B)2 U0
C)4 U0
D)The change in temperature must also be known to answer this question.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
65
In a physics experiment a pulsed electron beam is fired at a target.Each pulse lasts 60.0 ns,and there are 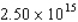 electrons in each pulse.Each electron in a pulse travels with a speed of
electrons in each pulse.Each electron in a pulse travels with a speed of 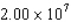 m/s.What is the impulse delivered to the target during one pulse if all the electrons are reflected elastically by the target?
m/s.What is the impulse delivered to the target during one pulse if all the electrons are reflected elastically by the target?
A)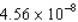 N·s
N·s
B)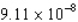 N·s
N·s
C)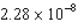 N·s
N·s
D)The impulse is more than double the largest value given in the other answers.
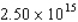 electrons in each pulse.Each electron in a pulse travels with a speed of
electrons in each pulse.Each electron in a pulse travels with a speed of 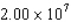 m/s.What is the impulse delivered to the target during one pulse if all the electrons are reflected elastically by the target?
m/s.What is the impulse delivered to the target during one pulse if all the electrons are reflected elastically by the target?A)
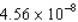 N·s
N·sB)
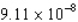 N·s
N·sC)
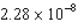 N·s
N·sD)The impulse is more than double the largest value given in the other answers.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
66
Different units can be used for length: m and cm,and of these two,m is the larger by a factor of 100.Different units can also be used for R: (1)J/mol*K, (2)L*atm/mol*K,and (3)(N/m2)*m3/mol*K.Which of these units for R is the largest? Hint: When expressing R in each of these units,which expression has the lowest numerical factor? (1L = 10-3 m3,1 atm = 1.01 x 105 Pa)
A)1
B)2
C)3
D)They are all equal.
A)1
B)2
C)3
D)They are all equal.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
67
Metal lids on glass jars can often be loosened by running them under hot water.Why is this?
A)The hot water is a lubricant.
B)The metal and glass expand due to the heating,and the glass being of smaller radius expands less than the metal.
C)The metal has a higher coefficient of thermal expansion than glass so the metal expands more than the glass thus loosening the connection.
D)This is just folklore.
A)The hot water is a lubricant.
B)The metal and glass expand due to the heating,and the glass being of smaller radius expands less than the metal.
C)The metal has a higher coefficient of thermal expansion than glass so the metal expands more than the glass thus loosening the connection.
D)This is just folklore.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
68
John rapidly pulls a plunger out of a cylinder.As the plunger moves away,the gas molecules bouncing elastically off the plunger are:
A)rebounding at a higher speed than they would have if the plunger weren't removed.
B)rebounding at a lower speed than they would have if the plunger weren't removed.
C)rebounding at the same speed as they would have if the plunger weren't removed.
D)Whether they speed up or slow down depends on how fast the plunger is removed.
A)rebounding at a higher speed than they would have if the plunger weren't removed.
B)rebounding at a lower speed than they would have if the plunger weren't removed.
C)rebounding at the same speed as they would have if the plunger weren't removed.
D)Whether they speed up or slow down depends on how fast the plunger is removed.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
69
The sulfur hexafluoride molecule consists of one sulfur atom and six fluorine atoms.The atomic masses of sulfur and fluorine are 32.0 u and 19.0 u respectively.One mole of this very heavy gas has what mass?
A)32 g
B)51 g
C)146 g
D)608 g
A)32 g
B)51 g
C)146 g
D)608 g

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
70
The ideal gas law treats gas as consisting of:
A)atoms.
B)molecules.
C)chemicals.
D)bubbles.
A)atoms.
B)molecules.
C)chemicals.
D)bubbles.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
71
A room has a volume of 60 m3 and is filled with air of an average molecular mass of 29 u.What is the mass of the air in the room at a pressure of 1.0 atm and temperature of 22 C? R = 0.082 L*atm/mol*K
A)2.4 kg
B)2 400 kg
C)72 kg
D)700 kg
A)2.4 kg
B)2 400 kg
C)72 kg
D)700 kg

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
72
For an ideal gas of a given mass,if the pressure remains the same and the volume increases:
A)the average kinetic energy of the molecules decreases.
B)the average kinetic energy of the molecules stays the same.
C)the average kinetic energy of the molecules increases.
D)Nothing can be determined about the molecular kinetic energy.
A)the average kinetic energy of the molecules decreases.
B)the average kinetic energy of the molecules stays the same.
C)the average kinetic energy of the molecules increases.
D)Nothing can be determined about the molecular kinetic energy.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
73
If the temperature of an ideal gas contained in a box is increased:
A)the average velocity of the molecules in the box will be increased.
B)the average speed of the molecules in the box will be increased.
C)the distance between molecules in the box will be increased.
D)all of the above.
A)the average velocity of the molecules in the box will be increased.
B)the average speed of the molecules in the box will be increased.
C)the distance between molecules in the box will be increased.
D)all of the above.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
74
Two ideal gases,X and Y,are thoroughly mixed and at thermal equilibrium in a single container.The molecular mass of X is 9 times that of Y.What is the ratio of root-mean-square velocities of the two gases,vX,rms /vY,rms?
A)9/1
B)3/1
C)1/3
D)1/9
A)9/1
B)3/1
C)1/3
D)1/9

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
75
A tank with a volume of 0.150 m3 contains 27.0 C helium gas at a pressure of 100 atm.How many balloons can be blown up if each filled balloon is a sphere 30.0 cm in diameter at 27.0 C and absolute pressure of 1.20 atm? Assume all the helium is transferred to the balloons.
A)963 balloons
B)884 balloons
C)776 balloons
D)598 balloons
A)963 balloons
B)884 balloons
C)776 balloons
D)598 balloons

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
76
Evaporation cools the liquid that is left behind because the molecules that leave the liquid during evaporation:
A)have kinetic energy.
B)have greater than average speed.
C)have broken the bonds that held them in the liquid.
D)create vapor pressure.
A)have kinetic energy.
B)have greater than average speed.
C)have broken the bonds that held them in the liquid.
D)create vapor pressure.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
77
A single pulse of monoenergetic protons is fired at a small target,and all the protons are absorbed.The speed of each of the protons 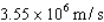 .The average pressure on the target during this pulse is
.The average pressure on the target during this pulse is 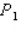 .The experiment is repeated,but this time the kinetic energy of the protons is doubled,the area of the target is doubled,and the duration of the pulse is doubled although the pulse contains the same number of protons as in the first procedure.What is the average pressure on the target during the second pulse?
.The experiment is repeated,but this time the kinetic energy of the protons is doubled,the area of the target is doubled,and the duration of the pulse is doubled although the pulse contains the same number of protons as in the first procedure.What is the average pressure on the target during the second pulse?
A)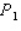
B)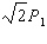
C)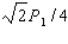
D)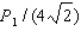
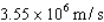 .The average pressure on the target during this pulse is
.The average pressure on the target during this pulse is 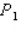 .The experiment is repeated,but this time the kinetic energy of the protons is doubled,the area of the target is doubled,and the duration of the pulse is doubled although the pulse contains the same number of protons as in the first procedure.What is the average pressure on the target during the second pulse?
.The experiment is repeated,but this time the kinetic energy of the protons is doubled,the area of the target is doubled,and the duration of the pulse is doubled although the pulse contains the same number of protons as in the first procedure.What is the average pressure on the target during the second pulse?A)
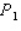
B)
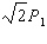
C)
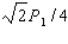
D)
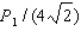

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
78
A pulsed proton beam is fired at a target.Each pulse lasts 45.0 ns,and there are 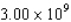 protons in each pulse,each proton having a speed of
protons in each pulse,each proton having a speed of 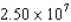 m/s.All the protons hit a circular area of
m/s.All the protons hit a circular area of 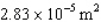 ,called the beam spot.What is the average pressure on the beam spot during a pulse if all the protons are absorbed by the target?
,called the beam spot.What is the average pressure on the beam spot during a pulse if all the protons are absorbed by the target?
A)250 Pa
B)98.4 Pa
C)197 Pa
D)49.2Pa
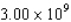 protons in each pulse,each proton having a speed of
protons in each pulse,each proton having a speed of 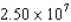 m/s.All the protons hit a circular area of
m/s.All the protons hit a circular area of 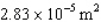 ,called the beam spot.What is the average pressure on the beam spot during a pulse if all the protons are absorbed by the target?
,called the beam spot.What is the average pressure on the beam spot during a pulse if all the protons are absorbed by the target?A)250 Pa
B)98.4 Pa
C)197 Pa
D)49.2Pa

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
79
What is the root-mean-square speed of chlorine gas molecules at a temperature of 320 K? (R = 8.31 J/mol*K,NA = 6.02 x 1023,and the molecular mass of Cl2 = 71)
A)1.7 x 102 m/s
B)3.4 x 102 m/s
C)0.8 x 104 m/s
D)1.1 x 105 m/s
A)1.7 x 102 m/s
B)3.4 x 102 m/s
C)0.8 x 104 m/s
D)1.1 x 105 m/s

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck
80
Consider two containers with the same volume and temperature.Container One holds "dry" air--a mixture of nitrogen and oxygen.Container Two holds "moist" air.The "moist" air has the same ratio of nitrogen to oxygen molecules,but also contains water vapor.According to the ideal gas law,if the pressures are equal,the weight of the gas in Container One will be:
A)lighter than the gas inside the second container.
B)equal to the weight of the gas in the second container.
C)heavier than the gas inside the second container.
D)all the above are incorrect because the pressures cannot be equal.
A)lighter than the gas inside the second container.
B)equal to the weight of the gas in the second container.
C)heavier than the gas inside the second container.
D)all the above are incorrect because the pressures cannot be equal.

Unlock Deck
Unlock for access to all 84 flashcards in this deck.
Unlock Deck
k this deck



