Deck 22: Organic and Biological Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/164
Play
Full screen (f)
Deck 22: Organic and Biological Molecules
1
Name the following: 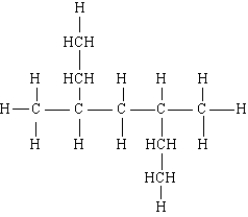
A)2,4-diethylpentane
B)3,5-dimethylheptane
C)secondary ethylpentane
D)2,3-dimethyl-2,3-diethylpropane
E)none of these

A)2,4-diethylpentane
B)3,5-dimethylheptane
C)secondary ethylpentane
D)2,3-dimethyl-2,3-diethylpropane
E)none of these
3,5-dimethylheptane
2
Which of the following names is a correct one?
A)3-methyl-4-isopropylpentane
B)2-ethyl-4-tertiary-butylpentane
C)2,2,3,5-tetramethylheptane
D)t-butylethane
E)trans-1,2-dimethylethane
A)3-methyl-4-isopropylpentane
B)2-ethyl-4-tertiary-butylpentane
C)2,2,3,5-tetramethylheptane
D)t-butylethane
E)trans-1,2-dimethylethane
2,2,3,5-tetramethylheptane
3
How many isomers of C3H8 are there?
A)1
B)2
C)3
D)5
E)6
A)1
B)2
C)3
D)5
E)6
1
4
Name the following: 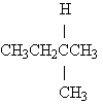
A)isopropane
B)methylpentane
C)methylbutane
D)n-pentane
E)dodecane

A)isopropane
B)methylpentane
C)methylbutane
D)n-pentane
E)dodecane

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
5
The compound below is the carbon skeleton (minus any hydrogen atoms)of 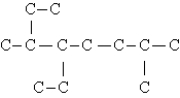 Which of these phrases could be used to describe this compound?
Which of these phrases could be used to describe this compound?
I.C12H26
II.a substituted octane
III.a compound with 3 tertiary carbons
IV.a compound with 3 secondary carbons
V.a compound with 2 isopropyl groups
A)I,II,III
B)II,III,IV
C)III,IV,V
D)II,IV,V
E)I,II,III,IV
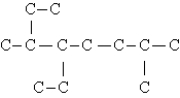 Which of these phrases could be used to describe this compound?
Which of these phrases could be used to describe this compound?I.C12H26
II.a substituted octane
III.a compound with 3 tertiary carbons
IV.a compound with 3 secondary carbons
V.a compound with 2 isopropyl groups
A)I,II,III
B)II,III,IV
C)III,IV,V
D)II,IV,V
E)I,II,III,IV

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following pairs is incorrect?
A)ethane - C2H4
B)pentane - C5H12
C)hexane - C6H14
D)heptane - C7H16
E)octane - C8H18
A)ethane - C2H4
B)pentane - C5H12
C)hexane - C6H14
D)heptane - C7H16
E)octane - C8H18

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
7
Which is a possible product of the chlorination of butane in the presence of light?
A)C4H9Cl
B)C4H8Cl
C)C4H10Cl2
D)C4H6Cl2
E)C4H9Cl2
A)C4H9Cl
B)C4H8Cl
C)C4H10Cl2
D)C4H6Cl2
E)C4H9Cl2

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
8
Name the following: 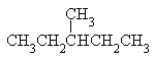
A)n-hexane
B)isohexane
C)1,2,3-trimethylpropane
D)methyl-diethylmethane
E)3-methylpentane
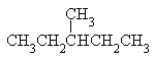
A)n-hexane
B)isohexane
C)1,2,3-trimethylpropane
D)methyl-diethylmethane
E)3-methylpentane

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
9
Name the following: 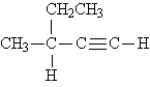
A)1-hexyne
B)2-ethynyl butane
C)2-ethyl-3-butyne
D)3-methyl-1-pentyne
E)3-methyl-4-pentyne

A)1-hexyne
B)2-ethynyl butane
C)2-ethyl-3-butyne
D)3-methyl-1-pentyne
E)3-methyl-4-pentyne

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following,upon reacting with oxygen,would form the greatest amount of carbon dioxide?
A)n-pentane
B)isopentane
C)neopentane
D)Two of the above would form equal amounts.
E)All (A-C)of the above would form equal amounts.
A)n-pentane
B)isopentane
C)neopentane
D)Two of the above would form equal amounts.
E)All (A-C)of the above would form equal amounts.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
11
Name the following: 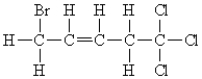
A)1,1,1-trichloro-5-bromo-3-pentene
B)1-bromo-5,5,5-trichloro-2-pentene
C)1,1,1-trichloro-5-bromo-2-pentene
D)1,1,1-trichloro-5-bromo-3-pentyne
E)none of these

A)1,1,1-trichloro-5-bromo-3-pentene
B)1-bromo-5,5,5-trichloro-2-pentene
C)1,1,1-trichloro-5-bromo-2-pentene
D)1,1,1-trichloro-5-bromo-3-pentyne
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
12
What is the compound whose carbon skeleton (minus any hydrogen atoms)appears below? 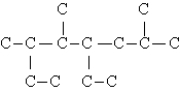
A)2,4-diethyl-3,6-dimethylheptane
B)2,5-dimethyl-4,6-diethylheptane
C)1,4-diethyl-3,6-dimethyl-tridecane
D)5-ethyl-3,6-trimethyloctane
E)4-ethyl-2,5,6-trimethyloctane
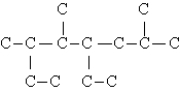
A)2,4-diethyl-3,6-dimethylheptane
B)2,5-dimethyl-4,6-diethylheptane
C)1,4-diethyl-3,6-dimethyl-tridecane
D)5-ethyl-3,6-trimethyloctane
E)4-ethyl-2,5,6-trimethyloctane

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
13
A student gave a molecule the following name: 2-methyl-4-t-butylpentane
However,the teacher pointed out that,although the molecule could be correctly drawn from this name,the name violates the IUPAC rules.What is the correct (IUPAC)name of the molecule?
A)2-t-butyl-4-methylpentane
B)2,2,3,5-tetramethylhexane
C)2,4,5,5-tetramethylhexane
D)1-sec-butyl-1,2,2-trimethylpentane
E)none of these
However,the teacher pointed out that,although the molecule could be correctly drawn from this name,the name violates the IUPAC rules.What is the correct (IUPAC)name of the molecule?
A)2-t-butyl-4-methylpentane
B)2,2,3,5-tetramethylhexane
C)2,4,5,5-tetramethylhexane
D)1-sec-butyl-1,2,2-trimethylpentane
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
14
In lecture,the professor named a molecule 4-ethylpentane.An alert student pointed out that although the correct structure could be drawn,the name did not follow systematic rules.What is the correct systematic name for the molecule?
A)2-ethylpentane
B)1-methyl-1-propylpropane
C)3-methylhexane
D)4-methylhexane
E)none of these
A)2-ethylpentane
B)1-methyl-1-propylpropane
C)3-methylhexane
D)4-methylhexane
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
15
A student gave a molecule the following name: 2-ethyl-3-methyl-5-isopropylhexane However,his TA pointed out that,although the molecule could be correctly drawn from this name,the name violates the systematic rules.What is the correct (systematic)name of the molecule?
A)3,4-dimethyl-6-isopropylheptane
B)2-isopropyl-4,5-dimethylheptane
C)3,4,6,7-tetramethyloctane
D)1,2-diethyl-3,6,7-trimethylheptane
E)2,3,5,6-tetramethyloctane
A)3,4-dimethyl-6-isopropylheptane
B)2-isopropyl-4,5-dimethylheptane
C)3,4,6,7-tetramethyloctane
D)1,2-diethyl-3,6,7-trimethylheptane
E)2,3,5,6-tetramethyloctane

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
16
Name the following: 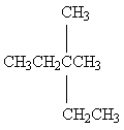
A)n-heptane
B)2-methyl-2-ethylbutane
C)3,3-dimethylpentane
D)2,2-diethylpropane
E)none of these

A)n-heptane
B)2-methyl-2-ethylbutane
C)3,3-dimethylpentane
D)2,2-diethylpropane
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
17
Cyclobutane has 109° bond angles like all alkanes.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
18
Name the following: CH3 -CH2 -CH3
A)ethane
B)propane
C)butane
D)pentane
E)hexane
A)ethane
B)propane
C)butane
D)pentane
E)hexane

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
19
Name the following: CH3-(CH2)6 -CH3
A)pentane
B)hexane
C)heptane
D)octane
E)nonane
A)pentane
B)hexane
C)heptane
D)octane
E)nonane

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
20
Why does octane have a higher boiling point than ethane,126°C versus -89°C?
A)Octane exhibits hydrogen bonding and ethane does not.
B)Octane has a higher vapor pressure than ethane.
C)Octane contains more double bonds than ethane.
D)Octane has stronger London dispersion forces than ethane.
E)At least two of the above are correct.
A)Octane exhibits hydrogen bonding and ethane does not.
B)Octane has a higher vapor pressure than ethane.
C)Octane contains more double bonds than ethane.
D)Octane has stronger London dispersion forces than ethane.
E)At least two of the above are correct.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
21
Name the following: 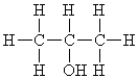
A)methyl alcohol
B)ethyl alcohol
C)propyl alcohol
D)isopropyl alcohol
E)butanol

A)methyl alcohol
B)ethyl alcohol
C)propyl alcohol
D)isopropyl alcohol
E)butanol

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
22
Consider the molecule trans-2-butene.Which statement is true?
A)The molecule has two bonds.
B)There is free rotation around every bond in the molecule.
C)Cis-2-butene is its structural isomer.
D)Carbon #2 exhibits sp2 hybridization.
E)None of the above.
A)The molecule has two bonds.
B)There is free rotation around every bond in the molecule.
C)Cis-2-butene is its structural isomer.
D)Carbon #2 exhibits sp2 hybridization.
E)None of the above.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
23
How many geometric isomers can be drawn for the following compound: CH3CH=CHCH2CH=C(CH3)2
A)2
B)3
C)4
D)5
E)6
A)2
B)3
C)4
D)5
E)6

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is known as rubbing alcohol?
A)methanol
B)ethanol
C)propanol
D)isopropanol
E)none of these
A)methanol
B)ethanol
C)propanol
D)isopropanol
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
25
Name the following: 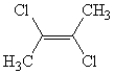
A)2-chloro-3-chloro-cis-2-butene
B)2,3-dichloro-cis-2-butene
C)2,3-dichloro-trans-2-butene
D)1-chloro-1-methyl-2-chloro-propene
E)2,3-dichloro-1-methyl-propene

A)2-chloro-3-chloro-cis-2-butene
B)2,3-dichloro-cis-2-butene
C)2,3-dichloro-trans-2-butene
D)1-chloro-1-methyl-2-chloro-propene
E)2,3-dichloro-1-methyl-propene

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is known as wood alcohol?
A)methanol
B)ethanol
C)propanol
D)isopropanol
E)none of these
A)methanol
B)ethanol
C)propanol
D)isopropanol
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
27
Hydrocarbons containing a carbon-carbon triple bond are called
A)alkynes
B)alkenes
C)cyclic alkanes
D)aldehydes
E)alkanes
A)alkynes
B)alkenes
C)cyclic alkanes
D)aldehydes
E)alkanes

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
28
How many different possible dimethylbenzenes exist?
A)2
B)3
C)4
D)5
E)6
A)2
B)3
C)4
D)5
E)6

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
29
One of the ingredients on a margarine container is listed as "polyunsaturated corn oil." This means that:
A)All the carbon bonds in the oil are single bonds.
B)Many of the polymer bonds are unsaturated.
C)All the carbon-carbon bonds are triple bonds.
D)Many of the carbon-carbon bonds are multiple bonds.
E)None of these.
A)All the carbon bonds in the oil are single bonds.
B)Many of the polymer bonds are unsaturated.
C)All the carbon-carbon bonds are triple bonds.
D)Many of the carbon-carbon bonds are multiple bonds.
E)None of these.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
30
Mothballs contain what aromatic hydrocarbon?
A)naphthalene
B)benzene
C)anthracene
D)phenanthrene
E)toluene
A)naphthalene
B)benzene
C)anthracene
D)phenanthrene
E)toluene

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
31
How many isomers are there of "dichloroethene"?
A)2
B)3
C)4
D)5
E)6
A)2
B)3
C)4
D)5
E)6

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following compounds can exhibit geometrical isomerism?
A)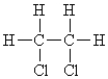
B)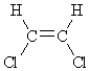
C)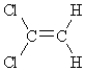
D)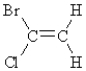
E)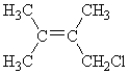
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
33
Chemical reactions involving alkanes in which hydrogen atoms are removed and the product is an unsaturated hydrocarbon are called
A)combustion reactions
B)dehydrogenation reactions
C)substitution reactions
D)addition reactions
E)polymerization reactions
A)combustion reactions
B)dehydrogenation reactions
C)substitution reactions
D)addition reactions
E)polymerization reactions

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is not a structural isomer of 1-pentene?
A)2-pentene
B)2-methyl-2-butene
C)cyclopentane
D)3-methyl-1-butene
E)1-methyl-cyclobutene
A)2-pentene
B)2-methyl-2-butene
C)cyclopentane
D)3-methyl-1-butene
E)1-methyl-cyclobutene

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
35
Consider the following four compounds: 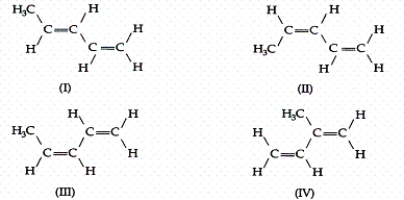 Which are the same molecule?
Which are the same molecule?
A)I and II
B)I and III
C)II and III
D)III and IV
E)I and IV
 Which are the same molecule?
Which are the same molecule?A)I and II
B)I and III
C)II and III
D)III and IV
E)I and IV

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
36
What is the most characteristic reaction of benzene?
A)oxidation
B)reduction
C)substitution
D)addition
E)addition and elimination
A)oxidation
B)reduction
C)substitution
D)addition
E)addition and elimination

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
37
Propane undergoes dehydrogenation.The product of this is
A)1-propene
B)2-propene
C)cis-1-propene
D)trans-1-propene
E)cis-2-propene
A)1-propene
B)2-propene
C)cis-1-propene
D)trans-1-propene
E)cis-2-propene

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
38
What is the compound represented by the following structure? 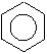
A)cyclohexene,C6H10
B)cyclohexane,C6H12
C)cyclohexatriene,C6H9
D)cyclohexatriene,C6H12
E)benzene,C6H6

A)cyclohexene,C6H10
B)cyclohexane,C6H12
C)cyclohexatriene,C6H9
D)cyclohexatriene,C6H12
E)benzene,C6H6

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
39
How many structural and geometrical isomers are there of chloropropene?
A)2
B)3
C)4
D)5
E)more than 5
A)2
B)3
C)4
D)5
E)more than 5

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
40
CH3C CCH2CH2Cl is named:
A)1-chloro-3-pentyne
B)5-chloro-2-pentene
C)1-acetylenyl-3-chloropropane
D)5-chloro-2-pentyne
E)1-chloro-3-pentene
A)1-chloro-3-pentyne
B)5-chloro-2-pentene
C)1-acetylenyl-3-chloropropane
D)5-chloro-2-pentyne
E)1-chloro-3-pentene

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
41
Classify the following molecule: 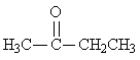
A)acid
B)aldehyde
C)amine
D)ketone
E)carbonyl
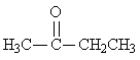
A)acid
B)aldehyde
C)amine
D)ketone
E)carbonyl

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following will yield a carboxylic acid upon oxidation?
A)a secondary alcohol
B)an aldehyde
C)a cycloalkane
D)a ketone
E)tertiary alcohol
A)a secondary alcohol
B)an aldehyde
C)a cycloalkane
D)a ketone
E)tertiary alcohol

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
43
What alcohols have the greatest commercial value?
A)methanol and ethanol
B)methanol and phenol
C)ethanol and phenol
D)1-propanol and ethanol
E)1-propanol and methanol
A)methanol and ethanol
B)methanol and phenol
C)ethanol and phenol
D)1-propanol and ethanol
E)1-propanol and methanol

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
44
Which molecule is an ether?
A)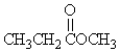
B)CH3CH2OCH3
C)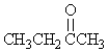
D)CH3CH2NH2
E)none of these
A)
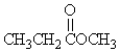
B)CH3CH2OCH3
C)
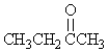
D)CH3CH2NH2
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is found in beverages such as wine?
A)methanol
B)ethanol
C)propanol
D)isopropanol
E)none of these
A)methanol
B)ethanol
C)propanol
D)isopropanol
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following becomes more soluble in water upon addition of NaOH?
A)an amine
B)a carboxylic acid
C)an aromatic hydrocarbon
D)an alkane
E)two of these
A)an amine
B)a carboxylic acid
C)an aromatic hydrocarbon
D)an alkane
E)two of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
47
Oxidation of a primary alcohol results in a(n)__________ and oxidation of a secondary alcohol results in a(n)_________.
A)carboxylic acid,amine
B)aldehyde,ketone
C)ester,ether
D)ketone,aldehyde
E)amine,carboxylic acid
A)carboxylic acid,amine
B)aldehyde,ketone
C)ester,ether
D)ketone,aldehyde
E)amine,carboxylic acid

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
48
Classify the following molecule: 
A)primary alcohol
B)secondary alcohol
C)tertiary alcohol
D)ether
E)phenol

A)primary alcohol
B)secondary alcohol
C)tertiary alcohol
D)ether
E)phenol

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
49
Classify the following molecule: 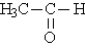
A)acid
B)aldehyde
C)amine
D)ketone
E)carbonyl

A)acid
B)aldehyde
C)amine
D)ketone
E)carbonyl

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
50
Identify the type of organic compound shown: 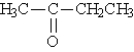
A)aldehyde
B)ester
C)amine
D)alcohol
E)none of these
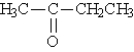
A)aldehyde
B)ester
C)amine
D)alcohol
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following yields a primary alcohol upon reduction?
A)a ketone
B)an alkene
C)an amine
D)an aldehyde
E)an ether
A)a ketone
B)an alkene
C)an amine
D)an aldehyde
E)an ether

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following has only one single C-O bond?
A)ketone
B)alcohol
C)ether
D)ester
E)aldehyde
A)ketone
B)alcohol
C)ether
D)ester
E)aldehyde

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
53
Identify the functional group present in the following organic compound: 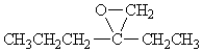
A)ester
B)aldehyde
C)ether
D)ketone
E)none of these
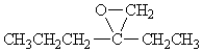
A)ester
B)aldehyde
C)ether
D)ketone
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
54
Which molecule is a ketone?
A)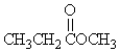
B)CH3CH2OCH3
C)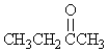
D)CH3CH2NH2
E)none of these
A)
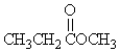
B)CH3CH2OCH3
C)
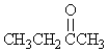
D)CH3CH2NH2
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
55
When C4H8 is treated with water and H2SO4, a tertiary alcohol is produced.Which of the following structures could represent C4H8 in this reaction?
A)CH3CH=CHCH3
B)CH3CH2CH=CH2
C)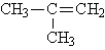
D)CH3CH2CH2CH3
E)none of these
A)CH3CH=CHCH3
B)CH3CH2CH=CH2
C)
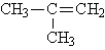
D)CH3CH2CH2CH3
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
56
Name the following: 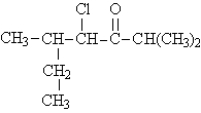
A)2-chloro-3-ethyl-1-isopropylbutanone
B)isopropyl-chloro,methylbutyl ketone
C)2-butyl,chloro,isobutanoyl methane
D)4-chloro-2,5-dimethyl-3-heptanone
E)3-methyl-4-chloro-1-isopropylpentanone

A)2-chloro-3-ethyl-1-isopropylbutanone
B)isopropyl-chloro,methylbutyl ketone
C)2-butyl,chloro,isobutanoyl methane
D)4-chloro-2,5-dimethyl-3-heptanone
E)3-methyl-4-chloro-1-isopropylpentanone

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
57
The boiling point of methanol is much higher than that of ethane.This is primarily due to
A)the difference in molar masses of methanol and ethane
B)the hydrogen bonding in methanol
C)the significant molecular size difference between methanol and ethane
D)the carbon oxygen double bond in the methanol
E)none of these
A)the difference in molar masses of methanol and ethane
B)the hydrogen bonding in methanol
C)the significant molecular size difference between methanol and ethane
D)the carbon oxygen double bond in the methanol
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
58
When the following organic compound is oxidized,what is the major organic product? 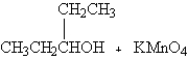
A)3-pentanoic acid
B)3-pentanol
C)3-pentanone
D)3-pentanal
E)No reaction takes place.
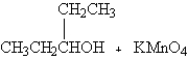
A)3-pentanoic acid
B)3-pentanol
C)3-pentanone
D)3-pentanal
E)No reaction takes place.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
59
Name the following: 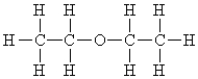
A)acetone
B)butyraldehyde
C)diethylketone
D)diethyl ether
E)none of these

A)acetone
B)butyraldehyde
C)diethylketone
D)diethyl ether
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
60
Identify the type of organic compound shown: 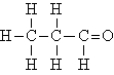
A)aldehyde
B)ester
C)amine
D)ketone
E)none of these
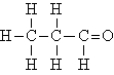
A)aldehyde
B)ester
C)amine
D)ketone
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following functional groups does not contain a doubly bonded oxygen (C=O)?
A)Aldehyde.
B)Carboxyl.
C)Ketone.
D)Carboxylic acid.
E)All contain a double bond.
A)Aldehyde.
B)Carboxyl.
C)Ketone.
D)Carboxylic acid.
E)All contain a double bond.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
62
Identify the secondary amine.
A)CH3NH2
B)(CH3)2NH
C)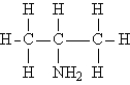
D)NH3
E)(CH3)3N
A)CH3NH2
B)(CH3)2NH
C)

D)NH3
E)(CH3)3N

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
63
Identify the type of organic compound shown: (CH3)3N
A)aldehyde
B)ester
C)amine
D)ketone
E)none of these
A)aldehyde
B)ester
C)amine
D)ketone
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
64
Name the following: 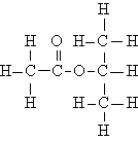
A)n-propyl acetate
B)isopropyl formate
C)isopropyl acetate
D)ethyl propanoate
E)none of these

A)n-propyl acetate
B)isopropyl formate
C)isopropyl acetate
D)ethyl propanoate
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
65
Referring to the structures below,which statement is true? I. 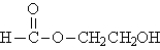 II.
II. 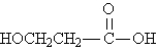 III.
III. 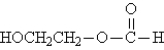
A)I and II have different molecular formulas.
B)I and III are structural isomers of each other.
C)II and III are stereoisomers of each other.
D)II and III are different conformations of the same compound.
E)I and III are the same compound.
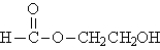 II.
II. 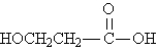 III.
III. 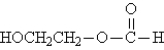
A)I and II have different molecular formulas.
B)I and III are structural isomers of each other.
C)II and III are stereoisomers of each other.
D)II and III are different conformations of the same compound.
E)I and III are the same compound.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
66
If you were to heat pentanoic acid and 2-butanol with a catalytic amount of strong acid,you would most likely discover in your flask:
A)a ketone
B)an ester
C)an amine
D)an alkane
E)an aldehyde
A)a ketone
B)an ester
C)an amine
D)an alkane
E)an aldehyde

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
67
H2CCHCH2N(CH3)2 is
A)an alkyne and a secondary amine
B)an alkene and a primary amine
C)an alkene and a tertiary amine
D)an alkyne and a tertiary amine
E)none of these
A)an alkyne and a secondary amine
B)an alkene and a primary amine
C)an alkene and a tertiary amine
D)an alkyne and a tertiary amine
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
68
Identify the type of organic compound shown: 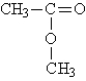
A)aldehyde
B)ester
C)amine
D)ketone
E)none of these

A)aldehyde
B)ester
C)amine
D)ketone
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
69
For which of the following compound(s)are cis and trans isomers possible?
A)2,3-dimethyl-2-butene
B)3-methyl-2-pentene
C)4,4-dimethylcyclohexanol
D)ortho-chlorotoluene
E)All can exhibit cis/trans isomers.
A)2,3-dimethyl-2-butene
B)3-methyl-2-pentene
C)4,4-dimethylcyclohexanol
D)ortho-chlorotoluene
E)All can exhibit cis/trans isomers.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
70
Classify the following molecule: 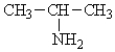
A)primary amine
B)secondary amine
C)tertiary amine
D)amino acid
E)peptide
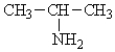
A)primary amine
B)secondary amine
C)tertiary amine
D)amino acid
E)peptide

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
71
What organic molecules have the general formula RCOOH?
A)esters
B)alcohols
C)carboxylic acids
D)ketones
E)aldehydes
A)esters
B)alcohols
C)carboxylic acids
D)ketones
E)aldehydes

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following has a double C-O bond and a single C-O bond?
A)ketone
B)ester
C)alcohol
D)amide
E)ether
A)ketone
B)ester
C)alcohol
D)amide
E)ether

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
73
What is the common name for acetylsalicylic acid?
A)orange juice
B)aspirin
C)acetone
D)bananas
E)vinegar
A)orange juice
B)aspirin
C)acetone
D)bananas
E)vinegar

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
74
Aspirin is formed via a(n)__________ reaction.
A)combustion
B)hydrogenation
C)addition
D)condensation
E)substitution
A)combustion
B)hydrogenation
C)addition
D)condensation
E)substitution

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
75
What organic compounds often have pleasant fruity odors?
A)ethers
B)alkynes
C)carboxylic acids
D)esters
E)amines
A)ethers
B)alkynes
C)carboxylic acids
D)esters
E)amines

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
76
A carboxylic acid will react with an alcohol to form a(n)__________ and a water molecule.
A)ester
B)amine
C)polymer
D)ketone
E)aldehyde
A)ester
B)amine
C)polymer
D)ketone
E)aldehyde

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
77
Which molecule is an amine?
A)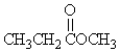
B)CH3CH2OCH3
C)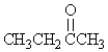
D)CH3CH2NH2
E)none of these
A)
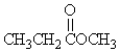
B)CH3CH2OCH3
C)
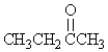
D)CH3CH2NH2
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following types of compounds must have an sp2-hybridized carbon center?
A)cyclic ethers
B)aldehydes
C)alcohols
D)alkynes
E)amines
A)cyclic ethers
B)aldehydes
C)alcohols
D)alkynes
E)amines

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following has an optical isomer?
A)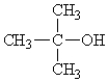
B)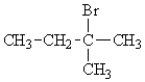
C)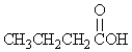
D)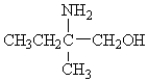
E)none of these
A)

B)

C)
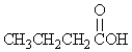
D)

E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
80
Which molecule is an ester?
A)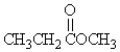
B)CH3CH2OCH3
C)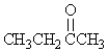
D)CH3CH2NH2
E)none of these
A)
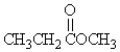
B)CH3CH2OCH3
C)
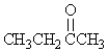
D)CH3CH2NH2
E)none of these

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck



