Deck 18: Electrochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/138
Play
Full screen (f)
Deck 18: Electrochemistry
1
How many electrons are transferred in the following reaction when it is balanced in acidic solution? SO32-(aq)+ MnO4-(aq) SO42-(aq)+ Mn2+(aq)
A)6
B)2
C)10
D)5
E)3
A)6
B)2
C)10
D)5
E)3
10
2
When the equation for the following reaction in basic solution is balanced,what is the sum of the coefficients? MnO2 + HO2- MnO4-
A)11
B)31
C)14
D)9
E)18
A)11
B)31
C)14
D)9
E)18
9
3
When the equation Cl2 Cl- + ClO3- (basic solution)is balanced using the smallest whole-number coefficients,the coefficient of OH- is:
A)1
B)12
C)3
D)4
E)6
A)1
B)12
C)3
D)4
E)6
6
4
The following reaction occurs in aqueous acid solution:
NO3- + I- IO3- + NO2
-In the balanced equation the coefficient of NO3- is:
A)2
B)3
C)4
D)5
E)6
NO3- + I- IO3- + NO2
-In the balanced equation the coefficient of NO3- is:
A)2
B)3
C)4
D)5
E)6

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
5
When the following reaction is balanced in acidic solution,what is the coefficient of I2? IO3- + I- I2
A)1
B)2
C)3
D)4
E)none of these
A)1
B)2
C)3
D)4
E)none of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
6
The following unbalanced equation represents a reaction that occurs in basic solution: MnO42- + C2O42- MnO2 + CO32-
How many moles of MnO42- are required to produce 1 mole of CO32-?
A)4
B)3
C)2
D)1
E)none of these
How many moles of MnO42- are required to produce 1 mole of CO32-?
A)4
B)3
C)2
D)1
E)none of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
7
When the equation for the following reaction in basic solution is balanced,what is the sum of the coefficients? MnO4-(aq)+ CN-(aq) MnO2(s)+ CNO-(aq)
A)13
B)8
C)10
D)20
E)11
A)13
B)8
C)10
D)20
E)11

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
8
Balance the following oxidation-reduction reaction using the half-reaction method. Cr2O72- + I2 Cr3+ + IO3-
In the balanced equation,the coefficient of water is:
A)4
B)17
C)11
D)7
E)6
In the balanced equation,the coefficient of water is:
A)4
B)17
C)11
D)7
E)6

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
9
The following reaction occurs in aqueous acid solution:
NO3- + I- IO3- + NO2
-In the balanced equation the coefficient of water is:
A)1
B)2
C)3
D)4
E)5
NO3- + I- IO3- + NO2
-In the balanced equation the coefficient of water is:
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
10
What is the oxidation state of Hg in Hg2Cl2?
A)+2
B)-1
C)-2
D)+1
E)0
A)+2
B)-1
C)-2
D)+1
E)0

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
11
Consider a galvanic cell based in the reaction Fe2+ + Cr2O72- Fe3+ + Cr3+ in acidic solution.
-What is the coefficient of Fe3+ in the balanced equation?
A)6
B)2
C)3
D)4
E)none of these
-What is the coefficient of Fe3+ in the balanced equation?
A)6
B)2
C)3
D)4
E)none of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
12
The reaction below occurs in basic solution.In the balanced equation,what is the sum of the coefficients? Zn + NO3- Zn(OH)42- + NH3
A)12
B)15
C)19
D)23
E)27
A)12
B)15
C)19
D)23
E)27

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
13
Ammonium metavandate reacts with sulfur dioxide in acidic solution as follows (hydrogen ions and H2O omitted): xVO3- + ySO2 xVO2+ + ySO42-
The ratio x : y is
A)1 : 1
B)1 : 2
C)2 : 1
D)1 : 3
E)3 : 1
The ratio x : y is
A)1 : 1
B)1 : 2
C)2 : 1
D)1 : 3
E)3 : 1

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
14
How many electrons are transferred in the following reaction? 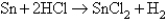
A)0
B)1
C)2
D)4
E)not enough information given
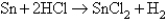
A)0
B)1
C)2
D)4
E)not enough information given

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
15
The following reaction occurs in basic solution: Ag+ + Cu Ag + Cu2+ When the equation is balanced,the sum of the coefficients is:
A)4
B)5
C)6
D)7
E)none of the above
A)4
B)5
C)6
D)7
E)none of the above

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
16
For the reaction of sodium bromide with chlorine gas to form sodium chloride and bromine,the appropriate half-reactions are (ox = oxidation and re = reduction):
A)ox: Cl2 + 2e- 2Cl-;re: 2Br- Br2 + 2e-
B)ox: 2Br- Br2 + 2e-;re: Cl2 + 2e- 2Cl-
C)ox: Cl + e- Cl-; re: Br Br- + e-
D)ox: Br + 2e- Br2-;re: 2Cl- Cl2 + 2e-
E)ox: 2Na+ + 2e- 2Na;re: 2Cl- Cl2 + 2e-
A)ox: Cl2 + 2e- 2Cl-;re: 2Br- Br2 + 2e-
B)ox: 2Br- Br2 + 2e-;re: Cl2 + 2e- 2Cl-
C)ox: Cl + e- Cl-; re: Br Br- + e-
D)ox: Br + 2e- Br2-;re: 2Cl- Cl2 + 2e-
E)ox: 2Na+ + 2e- 2Na;re: 2Cl- Cl2 + 2e-

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
17
The following questions refer to a galvanic cell that utilizes the following reaction (unbalanced): (AuCl4)-(aq)+ Cu(s) Au(s)+ Cl-(aq) + Cu2+(aq)
Determine the number of electrons transferred during the reaction (when balanced).
A)2
B)3
C)4
D)6
E)9
Determine the number of electrons transferred during the reaction (when balanced).
A)2
B)3
C)4
D)6
E)9

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
18
The following reaction occurs in basic solution: F2 + H2O O2 + F-
When the equation is balanced,the sum of the coefficients is:
A)10
B)11
C)12
D)13
E)none of these
When the equation is balanced,the sum of the coefficients is:
A)10
B)11
C)12
D)13
E)none of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
19
Given the following reaction in acidic media: Fe2+ + Cr2O72- Fe3+ + Cr3+
Answer the following question: The coefficient for water in the balanced reaction is:
A)1
B)3
C)5
D)7
E)none of these
Answer the following question: The coefficient for water in the balanced reaction is:
A)1
B)3
C)5
D)7
E)none of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
20
How many electrons are transferred in the following reaction? 2ClO3- + 12H+ + 10I- 5I2 + Cl2 + 6H2O
A)12
B)5
C)2
D)30
E)10
A)12
B)5
C)2
D)30
E)10

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
21
What is the value of Q,the reaction quotient,for this cell reaction?
A)6.7 1040
B)1.5 10-41
C)1.5 10-4
D)6.7 103
E)none of these
A)6.7 1040
B)1.5 10-41
C)1.5 10-4
D)6.7 103
E)none of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
22
The anode in a voltaic cell and in an electrolytic cell is
A)positive in both cells
B)the site of oxidation and of reduction,respectively
C)the site of reduction and of oxidation,respectively
D)the site of oxidation in both cells
E)the site of reduction in both cells
A)positive in both cells
B)the site of oxidation and of reduction,respectively
C)the site of reduction and of oxidation,respectively
D)the site of oxidation in both cells
E)the site of reduction in both cells

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
23
Which energy conversion shown below takes place in a galvanic cell?
A)electrical to chemical
B)chemical to electrical
C)mechanical to chemical
D)chemical to mechanical
E)mechanical to electrical
A)electrical to chemical
B)chemical to electrical
C)mechanical to chemical
D)chemical to mechanical
E)mechanical to electrical

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
24
A strip of copper is placed in a 1 M solution of copper nitrate and a strip of silver is placed in a 1 M solution of silver nitrate.The two metal strips are connected to a voltmeter by wires and a salt bridge connects the solutions.The following standard reduction potentials apply: Ag+(aq)+ e- Ag(s)
=+0.80 V
Cu2+(aq)+ 2e- Cu(s)
= +0.34 V
Which of the following statements is false?
A)Electrons flow in the external circuit from the copper electrode to the silver electrode.
B)The silver electrode increases in mass as the cell operates.
C)There is a net general movement of silver ions through the salt bridge to the copper half-cell.
D)Negative ions pass through the salt bridge from the silver half-cell to the copper half-cell.
E)Some positive copper ions pass through the salt bridge from the copper half-cell to the silver half-cell.
=+0.80 V
Cu2+(aq)+ 2e- Cu(s)
= +0.34 V
Which of the following statements is false?
A)Electrons flow in the external circuit from the copper electrode to the silver electrode.
B)The silver electrode increases in mass as the cell operates.
C)There is a net general movement of silver ions through the salt bridge to the copper half-cell.
D)Negative ions pass through the salt bridge from the silver half-cell to the copper half-cell.
E)Some positive copper ions pass through the salt bridge from the copper half-cell to the silver half-cell.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
25
Refer to the galvanic cell below (the contents of each half-cell are written beneath each compartment): 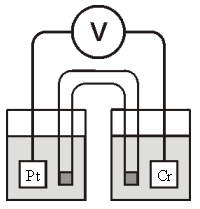 0.10 M MnO4- 0.40 M Cr3+
0.10 M MnO4- 0.40 M Cr3+
0.20 M Mn2+ 0.30 M Cr2O72-
0.010 M H+ 0.010 M H+
The standard reduction potentials are as follows:
MnO4- + 8H+ + 5e- Mn2+ + 4H2O, = 1.51 V
Cr2O72- + 14H+ + 6e- 2Cr3+ + 7H2O, =1.33 V
-When current is allowed to flow,which species is oxidized?
A)Cr2O72-
B)Cr3+
C)MnO4-
D)Mn2+
E)H+
 0.10 M MnO4- 0.40 M Cr3+
0.10 M MnO4- 0.40 M Cr3+0.20 M Mn2+ 0.30 M Cr2O72-
0.010 M H+ 0.010 M H+
The standard reduction potentials are as follows:
MnO4- + 8H+ + 5e- Mn2+ + 4H2O, = 1.51 V
Cr2O72- + 14H+ + 6e- 2Cr3+ + 7H2O, =1.33 V
-When current is allowed to flow,which species is oxidized?
A)Cr2O72-
B)Cr3+
C)MnO4-
D)Mn2+
E)H+

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following reactions is possible at the anode of a galvanic cell?
A)Zn Zn2+ + 2e-
B)Zn2+ + 2e- Zn
C)Zn2+ + Cu Zn + Cu2+
D)Zn + Cu2+ Zn2+ + Cu
E)two of these
A)Zn Zn2+ + 2e-
B)Zn2+ + 2e- Zn
C)Zn2+ + Cu Zn + Cu2+
D)Zn + Cu2+ Zn2+ + Cu
E)two of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is the best reducing agent? Cl2 + 2e- 2Cl-
= 1.36 V
Mg2+ + 2e- Mg
= -2.37 V
2H+ + 2e- H2
= 0.00 V
A)Cl2
B)H2
C)Mg
D)Mg2+
E)Cl-
= 1.36 V
Mg2+ + 2e- Mg
= -2.37 V
2H+ + 2e- H2
= 0.00 V
A)Cl2
B)H2
C)Mg
D)Mg2+
E)Cl-

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
28
What is the oxidation state of Cr in Cr2O72-?
A)+7
B)+6
C)+12
D)-1
E)-2
A)+7
B)+6
C)+12
D)-1
E)-2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
29
Consider a galvanic cell based in the reaction Fe2+ + Cr2O72- Fe3+ + Cr3+ in acidic solution.
-Calculate the voltage of the standard cell carrying out this reaction.
A)0
B)0.21 V
C)-0.21 V
D)0.56 V
E)-0.56 V
-Calculate the voltage of the standard cell carrying out this reaction.
A)0
B)0.21 V
C)-0.21 V
D)0.56 V
E)-0.56 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is true for the cell shown here? Zn(s)| Zn2+(aq)|| Cr3+(aq)| Cr(s)
A)The electrons flow from the cathode to the anode.
B)The electrons flow from the zinc to the chromium.
C)The electrons flow from the chromium to the zinc.
D)The chromium is oxidized.
E)The zinc is reduced.
A)The electrons flow from the cathode to the anode.
B)The electrons flow from the zinc to the chromium.
C)The electrons flow from the chromium to the zinc.
D)The chromium is oxidized.
E)The zinc is reduced.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
31
Consider the galvanic cell shown below (the contents of each half-cell are written beneath each compartment): 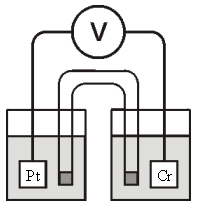 0.50 M Br2 0.20 M Cr3+ 0.10 M Br-
0.50 M Br2 0.20 M Cr3+ 0.10 M Br-
The standard reduction potentials are as follows:
Cr3+(aq)+ 3e- Cr(s) =-0.73 V
Br2(aq)+ 2e- 2Br-(aq) = +1.09 V
Which of the following statements about this cell is false?
A)This is a galvanic cell.
B)Electrons flow from the Pt electrode to the Cr electrode.
C)Reduction occurs at the Pt electrode.
D)The cell is not at standard conditions.
E)To complete the circuit,cations migrate into the left half-cell and anions migrate into the right half-cell from the salt bridge.
 0.50 M Br2 0.20 M Cr3+ 0.10 M Br-
0.50 M Br2 0.20 M Cr3+ 0.10 M Br-The standard reduction potentials are as follows:
Cr3+(aq)+ 3e- Cr(s) =-0.73 V
Br2(aq)+ 2e- 2Br-(aq) = +1.09 V
Which of the following statements about this cell is false?
A)This is a galvanic cell.
B)Electrons flow from the Pt electrode to the Cr electrode.
C)Reduction occurs at the Pt electrode.
D)The cell is not at standard conditions.
E)To complete the circuit,cations migrate into the left half-cell and anions migrate into the right half-cell from the salt bridge.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
32
Which statement is always true of the cathode in an electrochemical cell?
A)It is considered the "negative" electrode.
B)It is considered the "positive" electrode.
C)Reduction occurs here.
D)Metal is plated out here.
E)Negative ions flow toward the cathode.
A)It is considered the "negative" electrode.
B)It is considered the "positive" electrode.
C)Reduction occurs here.
D)Metal is plated out here.
E)Negative ions flow toward the cathode.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
33
Consider the galvanic cell shown below (the contents of each half-cell are written beneath each compartment): 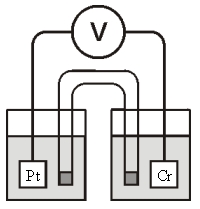 0.50 M Br2 0.20 M Cr3+ 0.10 M Br-
0.50 M Br2 0.20 M Cr3+ 0.10 M Br-
The standard reduction potentials are as follows:
Cr3+(aq)+ 3e- Cr(s) =-0.727 V
Br2(aq)+ 2e- 2Br-(aq) = +1.090 V
What is for this cell?
A)1.817 V
B)0.363 V
C)-0.363 V
D)4.724 V
E)1.316 V
 0.50 M Br2 0.20 M Cr3+ 0.10 M Br-
0.50 M Br2 0.20 M Cr3+ 0.10 M Br-The standard reduction potentials are as follows:
Cr3+(aq)+ 3e- Cr(s) =-0.727 V
Br2(aq)+ 2e- 2Br-(aq) = +1.090 V
What is for this cell?
A)1.817 V
B)0.363 V
C)-0.363 V
D)4.724 V
E)1.316 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is the strongest oxidizing agent? MnO4- + 4H+ + 3e- MnO2 + 2H2O
= 1.68 V
I2 + 2e- 2I-
= 0.54 V
Zn2+ + 2e- Zn
=-0.76 V
A)MnO4-
B)I2
C)Zn2+
D)Zn
E)MnO2
= 1.68 V
I2 + 2e- 2I-
= 0.54 V
Zn2+ + 2e- Zn
=-0.76 V
A)MnO4-
B)I2
C)Zn2+
D)Zn
E)MnO2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
35
Refer to the galvanic cell below (the contents of each half-cell are written beneath each compartment): 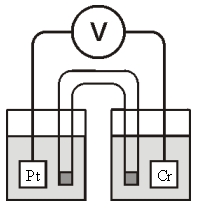 0.10 M MnO4- 0.40 M Cr3+ 0.20 M Mn2+ 0.30 M Cr2O72-
0.10 M MnO4- 0.40 M Cr3+ 0.20 M Mn2+ 0.30 M Cr2O72-
0.010 M H+ 0.010 M H+
The standard reduction potentials are as follows:
MnO4- + 8H+ + 5e- Mn2+ + 4H2O, = 1.506 V
Cr2O72- + 14H+ + 6e- 2Cr3+ + 7H2O, = 1.330 V
What is the value of cell?
A)-0.176
B)2.836
C)0.176
D)0.676
E)6.200
 0.10 M MnO4- 0.40 M Cr3+ 0.20 M Mn2+ 0.30 M Cr2O72-
0.10 M MnO4- 0.40 M Cr3+ 0.20 M Mn2+ 0.30 M Cr2O72-0.010 M H+ 0.010 M H+
The standard reduction potentials are as follows:
MnO4- + 8H+ + 5e- Mn2+ + 4H2O, = 1.506 V
Cr2O72- + 14H+ + 6e- 2Cr3+ + 7H2O, = 1.330 V
What is the value of cell?
A)-0.176
B)2.836
C)0.176
D)0.676
E)6.200

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
36
In which direction do electrons flow in the external circuit?
A)left to right
B)right to left
C)no current flows;the cell is at equilibrium
D)cannot be determined.
E)none of these
A)left to right
B)right to left
C)no current flows;the cell is at equilibrium
D)cannot be determined.
E)none of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
37
Which metal,Al or Ni could reduce Zn2+ to Zn(s)if placed in a Zn2+(aq)solution? Zn2+ + 2e- Zn
= -0.76 V
Al3+ + 3e- Al
= -1.66 V
Ni2+ + 2e- Ni
=-0.23 V
A)Al
B)Ni
C)Both Al and Ni would work.
D)Neither Al nor Ni would work.
E)Cannot be determined.
= -0.76 V
Al3+ + 3e- Al
= -1.66 V
Ni2+ + 2e- Ni
=-0.23 V
A)Al
B)Ni
C)Both Al and Ni would work.
D)Neither Al nor Ni would work.
E)Cannot be determined.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
38
You are told that metal X is a better reducing agent than metal Y.This must mean that:
A)X+ is a better oxidizing agent than Y+.
B)X+ is a better reducing agent than Y+.
C)Y is a better oxidizing agent than X.
D)Y+ is a better reducing agent than X+.
E)Y+ is a better oxidizing agent than X+.
A)X+ is a better oxidizing agent than Y+.
B)X+ is a better reducing agent than Y+.
C)Y is a better oxidizing agent than X.
D)Y+ is a better reducing agent than X+.
E)Y+ is a better oxidizing agent than X+.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
39
Refer to the galvanic cell below (the contents of each half-cell are written beneath each compartment):  0.10 M MnO4- 0.40 M Cr3+
0.10 M MnO4- 0.40 M Cr3+
0.20 M Mn2+ 0.30 M Cr2O72-
0.010 M H+ 0.010 M H+
The standard reduction potentials are as follows:
MnO4- + 8H+ + 5e- Mn2+ + 4H2O, = 1.51 V
Cr2O72- + 14H+ + 6e- 2Cr3+ + 7H2O, =1.33 V
-When current is allowed to flow,which species is reduced?
A)Cr2O72-
B)Cr3+
C)MnO4-
D)Mn2+
E)H+
 0.10 M MnO4- 0.40 M Cr3+
0.10 M MnO4- 0.40 M Cr3+0.20 M Mn2+ 0.30 M Cr2O72-
0.010 M H+ 0.010 M H+
The standard reduction potentials are as follows:
MnO4- + 8H+ + 5e- Mn2+ + 4H2O, = 1.51 V
Cr2O72- + 14H+ + 6e- 2Cr3+ + 7H2O, =1.33 V
-When current is allowed to flow,which species is reduced?
A)Cr2O72-
B)Cr3+
C)MnO4-
D)Mn2+
E)H+

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following species cannot function as an oxidizing agent?
A)S(s)
B)NO3-(aq)
C)Cr2O72-(aq)
D)I-(aq)
E)MnO4-(aq)
A)S(s)
B)NO3-(aq)
C)Cr2O72-(aq)
D)I-(aq)
E)MnO4-(aq)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
41
Determine the standard potential, ,of a cell that employs the reaction: Fe + Cu2+ Cu + Fe2+.
Reaction
(volts)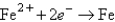 -0.441
-0.441 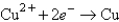 +0.340
+0.340
A)-0.101
B)-0.781
C)0.101
D)0.781
E)-0.202
Reaction
(volts)
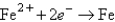 -0.441
-0.441 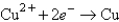 +0.340
+0.340A)-0.101
B)-0.781
C)0.101
D)0.781
E)-0.202

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
42
In the balanced cell reaction,what is the stoichiometric coefficient for H+?
A)5
B)6
C)30
D)22
E)2
A)5
B)6
C)30
D)22
E)2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
43
The following question refers to the following system: 3Ag(s)+ NO3-(aq)+ 4H+(aq) 3Ag+(aq)+ NO(g)+ 2H2O(l)
Anode reaction:
Ag Ag+(aq)+ e-
= -0.7990 V
Cathode reaction:
NO3-(aq)+ 4H+(aq)+ 3e- NO(g)+ 2H2O(l)
= 0.9644 V
Determine the standard cell potential.
A)-1.7634 V
B)0.1654 V
C)2.0942 V
D)3.5268 V
E)0.5878 V
Anode reaction:
Ag Ag+(aq)+ e-
= -0.7990 V
Cathode reaction:
NO3-(aq)+ 4H+(aq)+ 3e- NO(g)+ 2H2O(l)
= 0.9644 V
Determine the standard cell potential.
A)-1.7634 V
B)0.1654 V
C)2.0942 V
D)3.5268 V
E)0.5878 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
44
Consider the following electrode potentials: Mg2+ + 2e- \9\to\) Mg
= -2.37 V
V2+ + 2e- V
= -1.19 V
Cu2+ + e- Cu+
= 0.16 V
Which one of the reactions below will proceed spontaneously from left to right?
A)Mg2+ + V V2+ + Mg
B)Mg2+ + 2Cu+ 2Cu2+ + Mg
C)V2+ + 2Cu+ V + 2Cu2+
D)V + 2Cu2+ V2+ + 2Cu+
E)none of these
= -2.37 V
V2+ + 2e- V
= -1.19 V
Cu2+ + e- Cu+
= 0.16 V
Which one of the reactions below will proceed spontaneously from left to right?
A)Mg2+ + V V2+ + Mg
B)Mg2+ + 2Cu+ 2Cu2+ + Mg
C)V2+ + 2Cu+ V + 2Cu2+
D)V + 2Cu2+ V2+ + 2Cu+
E)none of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
45
You wish to plate out zinc metal from a zinc nitrate solution.Which metal,Al or Ni,could you place in the solution to accomplish this?
A)Al
B)Ni
C)Both Al and Ni would work.
D)Neither Al nor Ni would work.
E)Cannot be determined.
A)Al
B)Ni
C)Both Al and Ni would work.
D)Neither Al nor Ni would work.
E)Cannot be determined.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
46
Of Sn2+,Ag+,and/or Zn2+,which could be reduced by Cu?
A)Sn2+
B)Ag+
C)Zn2+
D)Two of them could be reduced by Cu.
E)All of them could be reduced by Cu.
A)Sn2+
B)Ag+
C)Zn2+
D)Two of them could be reduced by Cu.
E)All of them could be reduced by Cu.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
47
Choose the correct statement given the following information: 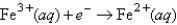
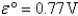
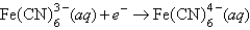
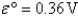
A)Fe2+(aq)is more likely to be oxidized than Fe2+ complexed to CN-.
B)Fe3+(aq)is more likely to be reduced than Fe3+ complexed to CN-.
C)Both A and B are true.
D)Complexation of Fe ions with CN- has no effect on their tendencies to become oxidized or reduced.
E)None of these is true.
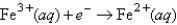
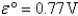
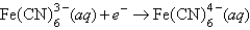
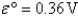
A)Fe2+(aq)is more likely to be oxidized than Fe2+ complexed to CN-.
B)Fe3+(aq)is more likely to be reduced than Fe3+ complexed to CN-.
C)Both A and B are true.
D)Complexation of Fe ions with CN- has no effect on their tendencies to become oxidized or reduced.
E)None of these is true.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following would be the best oxidizing agent?
A)Cl2
B)Fe
C)Na
D)Na+
E)F-
A)Cl2
B)Fe
C)Na
D)Na+
E)F-

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
49
What is the cell reaction for the voltaic cell Cr(s)| Cr3+(aq)|| Cl-(aq)| Cl2(g)| Pt?
A)Cr(s)+ 2Cl-(aq) Cl2(g)+ Cr3+(aq)
Cl2(g)+ Cr3+(aq)
B)2Cr3+(aq)+ 6Cl-(aq) 2Cr(s)+ 3Cl2(g)
2Cr(s)+ 3Cl2(g)
C)Cr(s)+ 3Cl2(g) Cr3+(s)+ 2Cl-(aq)
Cr3+(s)+ 2Cl-(aq)
D)2Cr(s)+ 3Cl2(g) 2Cr3+(aq)+ 6Cl-(aq)
2Cr3+(aq)+ 6Cl-(aq)
E)none of these
A)Cr(s)+ 2Cl-(aq)
 Cl2(g)+ Cr3+(aq)
Cl2(g)+ Cr3+(aq)B)2Cr3+(aq)+ 6Cl-(aq)
 2Cr(s)+ 3Cl2(g)
2Cr(s)+ 3Cl2(g)C)Cr(s)+ 3Cl2(g)
 Cr3+(s)+ 2Cl-(aq)
Cr3+(s)+ 2Cl-(aq)D)2Cr(s)+ 3Cl2(g)
 2Cr3+(aq)+ 6Cl-(aq)
2Cr3+(aq)+ 6Cl-(aq)E)none of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
50
How many electrons are transferred in the balanced reaction (i.e. ,what will be the value of n in the Nernst equation)?
A)5
B)6
C)30
D)22
E)2
A)5
B)6
C)30
D)22
E)2

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
51
What is the cell potential at 25°C as read on the digital voltmeter?
A)0.18 V
B)2.58 V
C)0.10 V
D)0.59 V
E)0.26 V
A)0.18 V
B)2.58 V
C)0.10 V
D)0.59 V
E)0.26 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
52
What is the balanced chemical equation corresponding to the following cell diagram? Na(s)| Na+(aq)|| Cu2+(aq)| Cu(s)
A)2Na(s)+ 2Na+(aq) Cu2+(aq)+ Cu(s)
B)Cu(s)+ 2Na+(aq) 2Na(s)+ Cu2+(aq)
C)2Na(s)+ Cu(s) 2Na+(aq)+ Cu2+(aq)
D)Cu2+(aq)+ Cu(s) 2Na(s)+ 2Na+(aq)
E)2Na(s)+ Cu2+(aq) 2Na+(aq)+ Cu(s)
A)2Na(s)+ 2Na+(aq) Cu2+(aq)+ Cu(s)
B)Cu(s)+ 2Na+(aq) 2Na(s)+ Cu2+(aq)
C)2Na(s)+ Cu(s) 2Na+(aq)+ Cu2+(aq)
D)Cu2+(aq)+ Cu(s) 2Na(s)+ 2Na+(aq)
E)2Na(s)+ Cu2+(aq) 2Na+(aq)+ Cu(s)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
53
Consider an electrochemical cell with a zinc electrode immersed in 1.0 M Zn2+ and a silver electrode immersed in 1.0 M Ag+. Zn2+ + 2e- Zn = -0.755 V
Ag+ + e- Ag = 0.800 V
Calculate for this cell.
A)0.045 V
B)-0.045 V
C)1.555 V
D)-1.555 V
E)none of these
Ag+ + e- Ag = 0.800 V
Calculate for this cell.
A)0.045 V
B)-0.045 V
C)1.555 V
D)-1.555 V
E)none of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
54
The following question refers to a galvanic cell that utilizes the following reaction (unbalanced): (AuCl4)-(aq)+ Cu(s) Au(s)+ Cl-(aq)+ Cu2+(aq)
Given the following information,determine the standard cell potential:
Species
Standard Reduction Potential (V)
Au3+(aq)
1.4980
Cu2+(aq)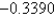
A)1.1590 V
B)1.8370 V
C)3.8160 V
D)0.8200 V
E)4.1550 V
Given the following information,determine the standard cell potential:
Species
Standard Reduction Potential (V)
Au3+(aq)
1.4980
Cu2+(aq)
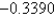
A)1.1590 V
B)1.8370 V
C)3.8160 V
D)0.8200 V
E)4.1550 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is the best reducing agent?
A)Cl2
B)H2
C)Mg
D)Mg2+
E)Cl-
A)Cl2
B)H2
C)Mg
D)Mg2+
E)Cl-

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
56
Copper will spontaneously reduce which of the following?
A)Fe2+ and Ag+
B)Fe2+
C)Ag+
D)Al3+
E)Fe2+ and Al3+
A)Fe2+ and Ag+
B)Fe2+
C)Ag+
D)Al3+
E)Fe2+ and Al3+

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
57
The galvanic cell described by Zn(s)| Zn2+(aq)| Cu2+(aq)| Cu(s)has a standard cell potential of 1.101 volts.Given that Zn(s) Zn2+(aq)+ 2e- has an oxidation potential of 0.764 volts,determine the reduction potential for 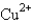 .
.
A)1.865 V
B)-1.865 V
C)-0.337 V
D)0.337 V
E)none of these
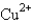 .
.A)1.865 V
B)-1.865 V
C)-0.337 V
D)0.337 V
E)none of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
58
A cell is set up with copper and lead electrodes in contact with CuSO4(aq)and Pb(NO3)2(aq),respectively,at 25°C.The standard reduction potentials are: Pb2+ + 2e- Pb = -0.13 V
Cu2+ + 2e- Cu = +0.34 V
If the Pb2+ and Cu2+ are each 1.0 M,the potential of the cell,in volts,is:
A)0.47 V
B)0.92 V
C)0.22 V
D)0.58 V
E)none of these
Cu2+ + 2e- Cu = +0.34 V
If the Pb2+ and Cu2+ are each 1.0 M,the potential of the cell,in volts,is:
A)0.47 V
B)0.92 V
C)0.22 V
D)0.58 V
E)none of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
59
The following has a potential of 0.92 V: 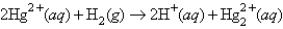 If the concentrations of the ions were 1.0 M and the pressure of H2 were 1.0 atm,then for the half-reaction
If the concentrations of the ions were 1.0 M and the pressure of H2 were 1.0 atm,then for the half-reaction 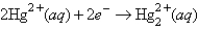 would be
would be
A)-0.92 V
B)-0.46 V
C)0.46 V
D)0.92 V
E)none of these
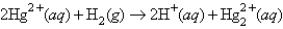 If the concentrations of the ions were 1.0 M and the pressure of H2 were 1.0 atm,then for the half-reaction
If the concentrations of the ions were 1.0 M and the pressure of H2 were 1.0 atm,then for the half-reaction 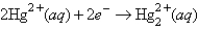 would be
would beA)-0.92 V
B)-0.46 V
C)0.46 V
D)0.92 V
E)none of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following cell diagrams represents a galvanic cell? ( red(Sn2+/Sn)= -0.138V, red(H+/H2)= 0.00 V)
A)Pt(s),H2(g)| H+(aq)|| Sn2+(aq)| Sn(s)
B)Sn(s)| Sn2+(aq)|| H+(aq)| H2(g),Pt(s)
C)Sn(s),H2(g)| H+(aq)|| Sn2+(aq)| Pt(s)
D)Pt(s)| Sn2+(aq)|| H+(aq)| H2(g),Sn(s)
E)Sn(s)| Pt(s)|| H+(aq),Sn2+(aq)| H2(g)
A)Pt(s),H2(g)| H+(aq)|| Sn2+(aq)| Sn(s)
B)Sn(s)| Sn2+(aq)|| H+(aq)| H2(g),Pt(s)
C)Sn(s),H2(g)| H+(aq)|| Sn2+(aq)| Pt(s)
D)Pt(s)| Sn2+(aq)|| H+(aq)| H2(g),Sn(s)
E)Sn(s)| Pt(s)|| H+(aq),Sn2+(aq)| H2(g)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
61
The following question refers to the following system: 3Ag(s)+ NO3-(aq)+ 4H+(aq) 3Ag+(aq)+ NO(g)+ 2H2O(l)
Anode reaction:
Ag Ag+(aq)+ 1e-
° = -0.7990 V
Cathode reaction:
NO3-(aq)+ 4H+(aq)+ 3e- NO(g)+ 2H2O(l)
° = 0.9636 V
Determine the equilibrium constant at 25°C.
A)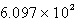
B)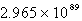
C)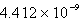
D)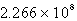
E)3.117
Anode reaction:
Ag Ag+(aq)+ 1e-
° = -0.7990 V
Cathode reaction:
NO3-(aq)+ 4H+(aq)+ 3e- NO(g)+ 2H2O(l)
° = 0.9636 V
Determine the equilibrium constant at 25°C.
A)
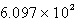
B)
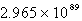
C)
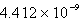
D)
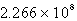
E)3.117

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
62
For a particular reaction in a galvanic (voltaic)cell S° is negative.Which of the following statements is true?
A)( ) will increase with an increase in temperature.
B)( ) will decrease with an increase in temperature.
C)( ) will not change when the temperature increases.
D)( G° > 0) for all temperatures.
E)None of the above statements is true.
A)( ) will increase with an increase in temperature.
B)( ) will decrease with an increase in temperature.
C)( ) will not change when the temperature increases.
D)( G° > 0) for all temperatures.
E)None of the above statements is true.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
63
A fuel cell designed to react grain alcohol with oxygen has the following net reaction: C2H5OH(l)+ 3O2(g) 2CO2(g)+ 3H2O(l)
The maximum work one mole of alcohol can yield by this process is 1320 kJ.What is the theoretical maximum voltage this cell can achieve?
A)0.760 V
B)1.14 V
C)2.01 V
D)2.28 V
E)13.7 V
The maximum work one mole of alcohol can yield by this process is 1320 kJ.What is the theoretical maximum voltage this cell can achieve?
A)0.760 V
B)1.14 V
C)2.01 V
D)2.28 V
E)13.7 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the following reduction potentials: Cu2+ + 2e- Cu = 0.342 V
Pb2+ + 2e- Pb = -0.130 V
For a galvanic cell employing the Cu,Cu2+ and Pb,Pb2+ couples,calculate the maximum amount of work that would accompany the reaction of one mole of lead under standard conditions.
A)-40.9 kJ
B)-45.5 kJ
C)-91.1 kJ
D)No work can be done.The system is at equilibrium.
E)None of these.
Pb2+ + 2e- Pb = -0.130 V
For a galvanic cell employing the Cu,Cu2+ and Pb,Pb2+ couples,calculate the maximum amount of work that would accompany the reaction of one mole of lead under standard conditions.
A)-40.9 kJ
B)-45.5 kJ
C)-91.1 kJ
D)No work can be done.The system is at equilibrium.
E)None of these.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following statements is true concerning the electrochemical cell depicted below? Ca | Ca2+(aq)|| K+(aq)| K
Ca2+(aq)+ 2e- Ca(s); = -2.87 V
K+(aq)+ e- K(s); = -2.93 V
A)The cell reaction is spontaneous with a standard cell potential of 0.06 V.
B)The cell reaction is nonspontaneous with a standard cell potential of -5.80 V.
C)The cell reaction is nonspontaneous with a standard cell potential of -0.06 V.
D)The cell reaction is spontaneous with a standard cell potential of 5.80 V.
E)The cell is at equilibrium.
Ca2+(aq)+ 2e- Ca(s); = -2.87 V
K+(aq)+ e- K(s); = -2.93 V
A)The cell reaction is spontaneous with a standard cell potential of 0.06 V.
B)The cell reaction is nonspontaneous with a standard cell potential of -5.80 V.
C)The cell reaction is nonspontaneous with a standard cell potential of -0.06 V.
D)The cell reaction is spontaneous with a standard cell potential of 5.80 V.
E)The cell is at equilibrium.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
66
Determine G° for a cell that utilizes the following reaction: Cl2(g)+ 2Br-(aq) 2Cl-(aq)+ Br2(l)
The standard reduction for the chlorine gas is 1.360 volts and the standard reduction for the bromine liquid is about 1.078 volts.
A)-470 kJ
B)-27.2 kJ
C)-235 kJ
D)-54.4 kJ
E)-23.9 kJ
The standard reduction for the chlorine gas is 1.360 volts and the standard reduction for the bromine liquid is about 1.078 volts.
A)-470 kJ
B)-27.2 kJ
C)-235 kJ
D)-54.4 kJ
E)-23.9 kJ

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following cell reactions would require the use of an inert electrode?
A)Co(s)+ 2Ag+(aq) Co2+(aq)+ 2Ag(s)
B)3Sr(s)+ 2Au3+(aq) 3Sr2+ + 2Au(s)
C)3Rb+(aq)+ Al(s) 3Rb(s)+ Al3+(aq)
D)Ba(s)+ 2MnO2(s)+ 2NH4+(aq) Ba2+(aq)+ Mn2O3(s)+ 2NH3(aq)+ H2O(l)
E)Sr(s)+ 2Ag+(aq) 2Ag(s)+ Sr2+(aq)
A)Co(s)+ 2Ag+(aq) Co2+(aq)+ 2Ag(s)
B)3Sr(s)+ 2Au3+(aq) 3Sr2+ + 2Au(s)
C)3Rb+(aq)+ Al(s) 3Rb(s)+ Al3+(aq)
D)Ba(s)+ 2MnO2(s)+ 2NH4+(aq) Ba2+(aq)+ Mn2O3(s)+ 2NH3(aq)+ H2O(l)
E)Sr(s)+ 2Ag+(aq) 2Ag(s)+ Sr2+(aq)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
68
The reduction potentials for Au3+ and Ni2+ are as follows: Au3+ + 3e- Au, = +1.50 V
Ni2+ + 2e- Ni, = -0.229 V
Calculate G° (at 25°C)for the reaction:
2Au3+ + 2Ni 3Ni2+ + 2Au
A)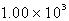 kJ
kJ
B)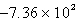 kJ
kJ
C)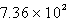 kJ
kJ
D)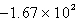 kJ
kJ
E)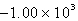 kJ
kJ
Ni2+ + 2e- Ni, = -0.229 V
Calculate G° (at 25°C)for the reaction:
2Au3+ + 2Ni 3Ni2+ + 2Au
A)
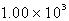 kJ
kJB)
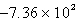 kJ
kJC)
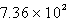 kJ
kJD)
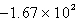 kJ
kJE)
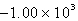 kJ
kJ
Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
69
For a certain reaction, H° = -74.4 kJ and S° = -227 J/K.If n = 3,calculate for the reaction at 25°C.
A)0.0233 V
B)0.491 V
C)0.277 V
D)0.0700 V
E)0.237 V
A)0.0233 V
B)0.491 V
C)0.277 V
D)0.0700 V
E)0.237 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
70
What is G° for the following electrochemical equation? ( °red(Ag+/Ag)= 0.800 V, °red(Cd2+/Cd)= -0.403 V) 2Ag(s)+ Cd2+(aq) 2Ag+(aq)+ Cd(s)
A)-232 kJ/mol
B)116 kJ/mol
C)232 kJ/mol
D)464 kJ/mol
E)-464 kJ/mol
A)-232 kJ/mol
B)116 kJ/mol
C)232 kJ/mol
D)464 kJ/mol
E)-464 kJ/mol

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
71
A common car battery consists of six identical cells,each of which carries out the reaction: Pb + PbO2 + 2HSO4- + 2H+ 2PbSO4 + 2H2O
The value of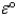 for such a cell is 2.039 V.Calculate G° at 25 °C for the reaction.
for such a cell is 2.039 V.Calculate G° at 25 °C for the reaction.
A)-196.7 kJ
B)-98.37 kJ
C)-393.5 kJ
D)-786.9 kJ
E)-590.2 kJ
The value of
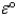 for such a cell is 2.039 V.Calculate G° at 25 °C for the reaction.
for such a cell is 2.039 V.Calculate G° at 25 °C for the reaction.A)-196.7 kJ
B)-98.37 kJ
C)-393.5 kJ
D)-786.9 kJ
E)-590.2 kJ

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
72
Under standard conditions,which of the following operations results in a spontaneous chemical reaction taking place?
A)A piece of aluminum metal is placed in an aqueous solution of potassium nitrate.
B)Iodine crystals are added to an aqueous solution of sodium chloride.
C)A piece of silver metal is placed in an aqueous solutions of copper(II)nitrate.
D)Chlorine gas is bubbled through an aqueous solution of sodium bromide.
E)At least two of the above (A-D)result in a spontaneous chemical reaction.
A)A piece of aluminum metal is placed in an aqueous solution of potassium nitrate.
B)Iodine crystals are added to an aqueous solution of sodium chloride.
C)A piece of silver metal is placed in an aqueous solutions of copper(II)nitrate.
D)Chlorine gas is bubbled through an aqueous solution of sodium bromide.
E)At least two of the above (A-D)result in a spontaneous chemical reaction.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
73
A galvanic cell consists of a left compartment with a tin electrode in contact with 0.1 M Sn(NO3)2(aq)and a right compartment with a lead electrode in contact with 1 10-3 M Pb(NO3)2(aq).The relevant reduction potentials are: Pb2+ + 2e- Pb
° = -0.13 V
Sn2+ + 2e- Sn
° = -0.14 V
When this cell is allowed to discharge spontaneously at 25°C,which of the following statements is true?
A)Electrons will flow from left to right through the wire.
B)Pb2+ ions will be reduced to Pb metal.
C)The concentration of Sn2+ ions in the left compartment will increase.
D)The tin electrode will be the cathode.
E)No noticeable change will occur,because the cell is at equilibrium.
° = -0.13 V
Sn2+ + 2e- Sn
° = -0.14 V
When this cell is allowed to discharge spontaneously at 25°C,which of the following statements is true?
A)Electrons will flow from left to right through the wire.
B)Pb2+ ions will be reduced to Pb metal.
C)The concentration of Sn2+ ions in the left compartment will increase.
D)The tin electrode will be the cathode.
E)No noticeable change will occur,because the cell is at equilibrium.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
74
Tables of standard reduction potentials are usually given at 25°C. depends on temperature.Which of the following equations describes the temperature dependence of ?
A)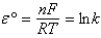
B)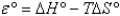
C)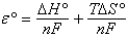
D)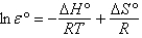
E)none of these
A)
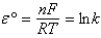
B)
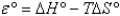
C)
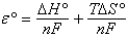
D)
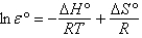
E)none of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
75
For a reaction in a voltaic cell both H° and S° are positive.Which of the following statements is true?
A)( cell) will increase with an increase in temperature.
B)( cell) will decrease with an increase in temperature.
C)( cell) will not change when the temperature increases.
D)( G° > 0) for all temperatures.
E)None of the above statements is true.
A)( cell) will increase with an increase in temperature.
B)( cell) will decrease with an increase in temperature.
C)( cell) will not change when the temperature increases.
D)( G° > 0) for all temperatures.
E)None of the above statements is true.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
76
Consider an electrochemical cell with a zinc electrode immersed in 1.0 M Zn2+ and a nickel electrode immersed in 0.10 M Ni2+.
Zn2+ + 2e- Zn
° = -0.76 V
Ni2+ + 2e- Ni
° = -0.23 V
-Calculate the concentration of Ni2+ if the cell is allowed to run to equilibrium at 25°C.
A)1.10 M
B)0.20 M
C)0.10 M
D)0 M
E)none of these
Zn2+ + 2e- Zn
° = -0.76 V
Ni2+ + 2e- Ni
° = -0.23 V
-Calculate the concentration of Ni2+ if the cell is allowed to run to equilibrium at 25°C.
A)1.10 M
B)0.20 M
C)0.10 M
D)0 M
E)none of these

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
77
Consider an electrochemical cell with a zinc electrode immersed in 1.0 M Zn2+ and a nickel electrode immersed in 0.10 M Ni2+.
Zn2+ + 2e- Zn
° = -0.76 V
Ni2+ + 2e- Ni
° = -0.23 V
-Calculate at 25°C for the cell shown below,given the following data: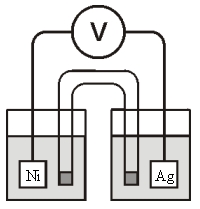
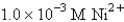
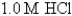
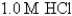
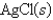
Ksp for AgCl = 1.6 10-10
A)0.83 V
B)0.54 V
C)1.01 V
D)2.98 V
E)cannot be determined from the data given
Zn2+ + 2e- Zn
° = -0.76 V
Ni2+ + 2e- Ni
° = -0.23 V
-Calculate at 25°C for the cell shown below,given the following data:

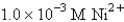
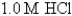
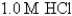
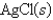
Ksp for AgCl = 1.6 10-10
A)0.83 V
B)0.54 V
C)1.01 V
D)2.98 V
E)cannot be determined from the data given

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
78
What is cell for the following electrochemical equation? ( red(Li+/Li)= -3.04 V red(Cu2+/Cu)= 0.342 V) 2Li(s)+ Cu2+(aq) 2Li+(aq)+ Cu(s)
A)-5.738 V
B)6.422 V
C)3.382 V
D)-3.382 V
E)-6.422 V
A)-5.738 V
B)6.422 V
C)3.382 V
D)-3.382 V
E)-6.422 V

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following statements is true concerning the electrochemical cell depicted below? Mg | Mg2+(aq)|| Cu2+(aq)| Cu
Mg2+(aq)+ 2e- Mg(s);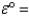 -2.38 V
-2.38 V
Cu2+(aq)+ 2e- Cu(s);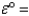 0.34 V
0.34 V
A)The cell reaction is spontaneous with a standard cell potential of 2.72 V.
B)The cell reaction is spontaneous with a standard cell potential of 2.04 V.
C)The cell reaction is nonspontaneous with a standard cell potential of -2.72 V.
D)The cell reaction is nonspontaneous with a standard cell potential of -2.04 V.
E)The cell is at equilibrium.
Mg2+(aq)+ 2e- Mg(s);
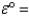 -2.38 V
-2.38 VCu2+(aq)+ 2e- Cu(s);
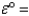 0.34 V
0.34 VA)The cell reaction is spontaneous with a standard cell potential of 2.72 V.
B)The cell reaction is spontaneous with a standard cell potential of 2.04 V.
C)The cell reaction is nonspontaneous with a standard cell potential of -2.72 V.
D)The cell reaction is nonspontaneous with a standard cell potential of -2.04 V.
E)The cell is at equilibrium.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
80
Given: 2H+(aq)+ 2e- H2(g);0.00 V
K+(aq)+ e- K(s);-2.93 V
F2(g)+ 2e- 2F-(aq);2.87 V
Al3+(aq)+ 3e- Al(s);-1.66 V
Pb2+(aq)+ 2e- Pb(s);-0.13 V
Under standard-state conditions,which is the strongest reducing agent?
A)H+
B)K
C)F-
D)Al3+
E)Pb2+
K+(aq)+ e- K(s);-2.93 V
F2(g)+ 2e- 2F-(aq);2.87 V
Al3+(aq)+ 3e- Al(s);-1.66 V
Pb2+(aq)+ 2e- Pb(s);-0.13 V
Under standard-state conditions,which is the strongest reducing agent?
A)H+
B)K
C)F-
D)Al3+
E)Pb2+

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck



