Deck 15: Acid-Base Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/94
Play
Full screen (f)
Deck 15: Acid-Base Equilibria
1
You have a 250.-mL sample of 1.28 M acetic acid (Ka = 1.8 10-5).Calculate the pH of the best buffer.
A)7.00
B)4.74
C)4.25
D)9.26
E)none of these
A)7.00
B)4.74
C)4.25
D)9.26
E)none of these
4.74
2
Which of the following will not produce a buffered solution?
A)100 mL of 0.1 M Na2CO3 and 50 mL of 0.1 M HCl
B)100 mL of 0.1 M NaHCO3 and 25 mL of 0.2 M HCl
C)100 mL of 0.1 M Na2CO3 and 75 mL of 0.2 M HCl
D)50 mL of 0.2 M Na2CO3 and 5 mL of 1.0 M HCl
E)100 mL of 0.1 M Na2CO3 and 50 mL of 0.1 M NaOH
A)100 mL of 0.1 M Na2CO3 and 50 mL of 0.1 M HCl
B)100 mL of 0.1 M NaHCO3 and 25 mL of 0.2 M HCl
C)100 mL of 0.1 M Na2CO3 and 75 mL of 0.2 M HCl
D)50 mL of 0.2 M Na2CO3 and 5 mL of 1.0 M HCl
E)100 mL of 0.1 M Na2CO3 and 50 mL of 0.1 M NaOH
100 mL of 0.1 M Na2CO3 and 50 mL of 0.1 M NaOH
3
Which of the following is true for a buffered solution?
A)The solution resists change in its [H+].
B)The solution will not change its pH very much even if a concentrated acid is added.
C)The solution will not change its pH very much even if a strong base is added.
D)Any H+ ions will react with a conjugate base of a weak acid already in solution.
E)All of these.
A)The solution resists change in its [H+].
B)The solution will not change its pH very much even if a concentrated acid is added.
C)The solution will not change its pH very much even if a strong base is added.
D)Any H+ ions will react with a conjugate base of a weak acid already in solution.
E)All of these.
All of these.
4
The following question refers to a 2.0-liter buffered solution created from 0.31 M NH3 (Kb = 1.8 10-5)and 0.26 M NH4F.When 0.10 mol of H+ ions is added to the solution what is the pH?
A)4.82
B)4.66
C)10.53
D)9.18
E)7.88
A)4.82
B)4.66
C)10.53
D)9.18
E)7.88

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
5
A 100.mL sample of 0.10 M HCl is mixed with 50.mL of 0.11 M NH3.What is the resulting pH? (Kb for NH3 = 1.8 10-5)
A)3.09
B)10.91
C)12.48
D)1.35
E)1.52
A)3.09
B)10.91
C)12.48
D)1.35
E)1.52

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
6
You have a 250.0-mL sample of 1.00 M acetic acid (Ka = 1.8 10-5).Calculate the pH after adding 0.0050 mol of NaOH to 1.0 liter of the best buffer.
A)7.05
B)2.41
C)3.54
D)4.78
E)none of these
A)7.05
B)2.41
C)3.54
D)4.78
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
7
Suppose a buffer solution is made from formic and (HCHO2)and sodium formate (NaCHO2).What is the net ionic equation for the reaction that occurs when a small amount of hydrochloric acid is added to the buffer?
A)H3O+(aq)+ OH-(aq) 2H2O(l)
B)H3O+(aq)+ HCHO2(aq) H2O(l)+ H2CHO2+(aq)
C)HCl(aq)+ OH-(aq) H2O(l)+ Cl-(aq)
D)HCl(aq)+ CHO2-(aq) HCHO2(aq)+ Cl-(aq)
E)H3O+(aq)+ CHO2-(aq) HCHO2(aq)+ H2O(l)
A)H3O+(aq)+ OH-(aq) 2H2O(l)
B)H3O+(aq)+ HCHO2(aq) H2O(l)+ H2CHO2+(aq)
C)HCl(aq)+ OH-(aq) H2O(l)+ Cl-(aq)
D)HCl(aq)+ CHO2-(aq) HCHO2(aq)+ Cl-(aq)
E)H3O+(aq)+ CHO2-(aq) HCHO2(aq)+ H2O(l)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
8
A weak acid,HF,is in solution with dissolved sodium fluoride,NaF.If HCl is added,which ion will react with the extra hydrogen ions from the HCl to keep the pH from changing?
A)OH-
B)Na+
C)F-
D)Na-
E)none of these
A)OH-
B)Na+
C)F-
D)Na-
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
9
What will happen if a small amount of hydrochloric acid is added to a 0.1 M solution of HF?
A)The percent ionization of HF will increase.
B)The percent ionization of HF will decrease.
C)The percent ionization of HF will remain unchanged.
D)Ka for HF will increase.
E)Ka for HF will decrease.
A)The percent ionization of HF will increase.
B)The percent ionization of HF will decrease.
C)The percent ionization of HF will remain unchanged.
D)Ka for HF will increase.
E)Ka for HF will decrease.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following mixtures would result in a buffered solution?
A)Mixing 100.0 mL of 0.100 M HCl with 100.0 mL of 0.100 M NaOH.
B)Mixing 100.0 mL of 0.100 M NH3 (Kb = 1.8 10-5)with 100.0 mL of 0.100 M NaOH.
C)Mixing 100.0 mL of 0.100 M HCl with 100.0 mL of 0.100 M NH3 (Kb = 1.8 10-5).
D)Mixing 50.0 mL of 0.100 M HCl with 100.0 mL of 0.100 M NH3 (Kb = 1.8 10-5).
E)At least two of the above mixtures would result in a buffered solution.
A)Mixing 100.0 mL of 0.100 M HCl with 100.0 mL of 0.100 M NaOH.
B)Mixing 100.0 mL of 0.100 M NH3 (Kb = 1.8 10-5)with 100.0 mL of 0.100 M NaOH.
C)Mixing 100.0 mL of 0.100 M HCl with 100.0 mL of 0.100 M NH3 (Kb = 1.8 10-5).
D)Mixing 50.0 mL of 0.100 M HCl with 100.0 mL of 0.100 M NH3 (Kb = 1.8 10-5).
E)At least two of the above mixtures would result in a buffered solution.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
11
For a solution equimolar in HCN and NaCN,which statement is false?
A)This is an example of the common ion effect.
B)The [H+] is larger than it would be if only the HCN was in solution.
C)The [H+] is equal to the Ka.
D)Addition of more NaCN will shift the acid dissociation equilibrium of HCN to the left.
E)Addition of NaOH will increase [CN-] and decrease [HCN].
A)This is an example of the common ion effect.
B)The [H+] is larger than it would be if only the HCN was in solution.
C)The [H+] is equal to the Ka.
D)Addition of more NaCN will shift the acid dissociation equilibrium of HCN to the left.
E)Addition of NaOH will increase [CN-] and decrease [HCN].

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
12
The following question refers to a 2.0-liter buffered solution created from 0.72 M NH3 (Kb = 1.8 10-5)and 0.26 M NH4F.What is the pH of this solution?
A)9.26
B)9.70
C)4.30
D)5.18
E)8.81
A)9.26
B)9.70
C)4.30
D)5.18
E)8.81

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
13
You have solutions of 0.200 M HNO2 and 0.200 M KNO2 (Ka for HNO2 = 4.00 10-4).A buffer of pH 3.000 is needed.What volumes of HNO2 and KNO2 are required to make 1 liter of buffered solution?
A)500 mL of each
B)286 mL HNO2;714 mL KNO2
C)413 mL HNO2;587 mL KNO2
D)714 mL HNO2;286 mL KNO2
E)587 mL HNO2;413 mL KNO2
A)500 mL of each
B)286 mL HNO2;714 mL KNO2
C)413 mL HNO2;587 mL KNO2
D)714 mL HNO2;286 mL KNO2
E)587 mL HNO2;413 mL KNO2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
14
You have a 250.-mL sample of 1.250 M acetic acid (Ka = 1.8 10-5).Assuming no volume change,how much NaOH must be added to make the best buffer?
A)6.25 g
B)12.5 g
C)16.3 g
D)21.3 g
E)none of these
A)6.25 g
B)12.5 g
C)16.3 g
D)21.3 g
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
15
You have a 250.0-mL sample of 1.00 M acetic acid (Ka = 1.8 10-5).Calculate the pH after adding 0.0040 mol HCl to 1.0 liter of the best buffer.
A)4.72
B)2.35
C)3.12
D)6.98
E)none of these
A)4.72
B)2.35
C)3.12
D)6.98
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
16
Suppose a buffer solution is made from formic acid,HCHO2,and sodium formate,NaCHO2.What is the net ionic equation for the reaction that occurs when a small amount of sodium hydroxide is added to the buffer?
A)NaOH(aq)+ H3O+(aq) Na+(aq)+ 2H2O(l)
B)H3O+(aq)+ OH-(aq) 2H2O(l)
C)OH-(aq)+ HCHO2(aq) CHO2-(aq)+ H2O(l)
D)NaOH(aq)+ HCHO2(aq) NaCHO2(aq)+ H2O(l)
E)Na+(aq)+ HCHO2(aq) NaH(aq)+ HCO2+(aq)
A)NaOH(aq)+ H3O+(aq) Na+(aq)+ 2H2O(l)
B)H3O+(aq)+ OH-(aq) 2H2O(l)
C)OH-(aq)+ HCHO2(aq) CHO2-(aq)+ H2O(l)
D)NaOH(aq)+ HCHO2(aq) NaCHO2(aq)+ H2O(l)
E)Na+(aq)+ HCHO2(aq) NaH(aq)+ HCO2+(aq)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
17
What will happen if a small amount of sodium hydroxide is added to a 0.1 M solution of ammonia?
A)Kb for ammonia will increase.
B)Kb for ammonia will decrease.
C)The percent ionization of ammonia will increase.
D)The percent ionization of ammonia will decrease.
E)The percent ionization of ammonia will remain unchanged.
A)Kb for ammonia will increase.
B)Kb for ammonia will decrease.
C)The percent ionization of ammonia will increase.
D)The percent ionization of ammonia will decrease.
E)The percent ionization of ammonia will remain unchanged.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
18
What combination of substances will give a buffered solution that has a pH of 5.05? (Assume each pair of substances is dissolved in 5.0 L of water. )(Kb for NH3 = 1.8 10-5;Kb for C5H5N = 1.7 10-9)
A)1.0 mole NH3 and 1.5 mole NH4Cl
B)1.5 mole NH3 and 1.0 mole NH4Cl
C)1.0 mole C5H5N and 1.5 mole C5H5NHCl
D)1.5 mole C5H5N and 1.0 mole C5H5NHCl
E)none of these
A)1.0 mole NH3 and 1.5 mole NH4Cl
B)1.5 mole NH3 and 1.0 mole NH4Cl
C)1.0 mole C5H5N and 1.5 mole C5H5NHCl
D)1.5 mole C5H5N and 1.0 mole C5H5NHCl
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
19
What is the percent dissociation of HNO2 when 0.064 g of sodium nitrite is added to 120.0 mL of a 0.057 M HNO2 solution? Ka for HNO2 is 4.0 10-4.
A)13%
B)0.30%
C)5.2%
D)0.075%
E)8.4%
A)13%
B)0.30%
C)5.2%
D)0.075%
E)8.4%

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
20
Calculate the [H+] in a solution that is 0.16 M in NaF and 0.25 M in HF.(Ka = 7.2 10-4)
A)7.2 10-4 M
B)1.6 M
C)1.1 10-3 M
D)0.20 M
E)4.6 10-4 M
A)7.2 10-4 M
B)1.6 M
C)1.1 10-3 M
D)0.20 M
E)4.6 10-4 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
21
Calculate the pH of a solution made by mixing 100.0 mL of 0.635 M NH3 with 100.0 mL of 0.100 M HCl.(Kb for NH3 = 1.8 10-5)
A)9.98
B)4.02
C)8.53
D)9.26
E)none of these
A)9.98
B)4.02
C)8.53
D)9.26
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
22
The following question refers to the following system: A 1.0-liter solution contains 0.25 M HF and 0.32 M NaF (Ka for HF is 7.2 10-4). If one adds 0.30 liters of 0.020 M KOH to the solution,what will be the change in pH?
A)0.02
B)3.27
C)0.13
D)-0.11
E)-0.28
A)0.02
B)3.27
C)0.13
D)-0.11
E)-0.28

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
23
You have two buffered solutions.Buffered solution 1 consists of 5.0 M HOAc and 5.0 M NaOAc;buffered solution 2 is made of 0.050 M HOAc and 0.050 M NaOAc.
How do the pHs of the buffered solutions compare?
A)The pH of buffered solution 1 is greater than that of buffered solution 2.
B)The pH of buffered solution 2 is greater than that of buffered solution 1.
C)The pH of buffered solution 1 is equal to that of buffered solution 2.
D)Cannot be determined without the Ka values.
E)None of these (A-D).
How do the pHs of the buffered solutions compare?
A)The pH of buffered solution 1 is greater than that of buffered solution 2.
B)The pH of buffered solution 2 is greater than that of buffered solution 1.
C)The pH of buffered solution 1 is equal to that of buffered solution 2.
D)Cannot be determined without the Ka values.
E)None of these (A-D).

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
24
Consider a solution consisting of the following two buffer systems: H2CO3 ![<strong>Consider a solution consisting of the following two buffer systems: H<sub>2</sub>CO<sub>3</sub> HCO<sub>3</sub><sup>-</sup> + H<sup>+ </sup>pK<sub>a</sub> = 6.4 H<sub>2</sub>PO<sub>4</sub><sup>-</sup> HPO<sub>4</sub><sup>2</sup><sup>-</sup> + H<sup>+ </sup>pK<sub>a</sub> = 7.2 At pH 6.4,which one of the following is true of the relative amounts of acid and conjugate base present?</strong> A)[H<sub>2</sub>CO<sub>3</sub>] > [HCO<sub>3</sub><sup>-</sup>] and [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] > [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] B)[H<sub>2</sub>CO<sub>3</sub>] = [HCO<sub>3</sub><sup>-</sup>] and [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] > [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] C)[H<sub>2</sub>CO<sub>3</sub>] = [HCO<sub>3</sub><sup>-</sup>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] D)[HCO<sub>3</sub><sup>-</sup>] > [H<sub>2</sub>CO<sub>3</sub>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] E)[H<sub>2</sub>CO<sub>3</sub>] > [HCO<sub>3</sub><sup>-</sup>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>]](https://d2lvgg3v3hfg70.cloudfront.net/TB6423/11eaa8f0_66e6_cb5c_93a6_8fd44e0f825b_TB6423_11.jpg) HCO3- + H+ pKa = 6.4
HCO3- + H+ pKa = 6.4
H2PO4-![<strong>Consider a solution consisting of the following two buffer systems: H<sub>2</sub>CO<sub>3</sub> HCO<sub>3</sub><sup>-</sup> + H<sup>+ </sup>pK<sub>a</sub> = 6.4 H<sub>2</sub>PO<sub>4</sub><sup>-</sup> HPO<sub>4</sub><sup>2</sup><sup>-</sup> + H<sup>+ </sup>pK<sub>a</sub> = 7.2 At pH 6.4,which one of the following is true of the relative amounts of acid and conjugate base present?</strong> A)[H<sub>2</sub>CO<sub>3</sub>] > [HCO<sub>3</sub><sup>-</sup>] and [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] > [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] B)[H<sub>2</sub>CO<sub>3</sub>] = [HCO<sub>3</sub><sup>-</sup>] and [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] > [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] C)[H<sub>2</sub>CO<sub>3</sub>] = [HCO<sub>3</sub><sup>-</sup>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] D)[HCO<sub>3</sub><sup>-</sup>] > [H<sub>2</sub>CO<sub>3</sub>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] E)[H<sub>2</sub>CO<sub>3</sub>] > [HCO<sub>3</sub><sup>-</sup>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>]](https://d2lvgg3v3hfg70.cloudfront.net/TB6423/11eaa8f0_66e6_cb5d_93a6_974fa267e285_TB6423_11.jpg) HPO42- + H+ pKa = 7.2
HPO42- + H+ pKa = 7.2
At pH 6.4,which one of the following is true of the relative amounts of acid and conjugate base present?
A)[H2CO3] > [HCO3-] and [H2PO4-] > [HPO42-]
B)[H2CO3] = [HCO3-] and [H2PO4-] > [HPO42-]
C)[H2CO3] = [HCO3-] and [HPO42-] > [H2PO4-]
D)[HCO3-] > [H2CO3] and [HPO42-] > [H2PO4-]
E)[H2CO3] > [HCO3-] and [HPO42-] > [H2PO4-]
![<strong>Consider a solution consisting of the following two buffer systems: H<sub>2</sub>CO<sub>3</sub> HCO<sub>3</sub><sup>-</sup> + H<sup>+ </sup>pK<sub>a</sub> = 6.4 H<sub>2</sub>PO<sub>4</sub><sup>-</sup> HPO<sub>4</sub><sup>2</sup><sup>-</sup> + H<sup>+ </sup>pK<sub>a</sub> = 7.2 At pH 6.4,which one of the following is true of the relative amounts of acid and conjugate base present?</strong> A)[H<sub>2</sub>CO<sub>3</sub>] > [HCO<sub>3</sub><sup>-</sup>] and [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] > [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] B)[H<sub>2</sub>CO<sub>3</sub>] = [HCO<sub>3</sub><sup>-</sup>] and [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] > [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] C)[H<sub>2</sub>CO<sub>3</sub>] = [HCO<sub>3</sub><sup>-</sup>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] D)[HCO<sub>3</sub><sup>-</sup>] > [H<sub>2</sub>CO<sub>3</sub>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] E)[H<sub>2</sub>CO<sub>3</sub>] > [HCO<sub>3</sub><sup>-</sup>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>]](https://d2lvgg3v3hfg70.cloudfront.net/TB6423/11eaa8f0_66e6_cb5c_93a6_8fd44e0f825b_TB6423_11.jpg) HCO3- + H+ pKa = 6.4
HCO3- + H+ pKa = 6.4H2PO4-
![<strong>Consider a solution consisting of the following two buffer systems: H<sub>2</sub>CO<sub>3</sub> HCO<sub>3</sub><sup>-</sup> + H<sup>+ </sup>pK<sub>a</sub> = 6.4 H<sub>2</sub>PO<sub>4</sub><sup>-</sup> HPO<sub>4</sub><sup>2</sup><sup>-</sup> + H<sup>+ </sup>pK<sub>a</sub> = 7.2 At pH 6.4,which one of the following is true of the relative amounts of acid and conjugate base present?</strong> A)[H<sub>2</sub>CO<sub>3</sub>] > [HCO<sub>3</sub><sup>-</sup>] and [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] > [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] B)[H<sub>2</sub>CO<sub>3</sub>] = [HCO<sub>3</sub><sup>-</sup>] and [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] > [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] C)[H<sub>2</sub>CO<sub>3</sub>] = [HCO<sub>3</sub><sup>-</sup>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] D)[HCO<sub>3</sub><sup>-</sup>] > [H<sub>2</sub>CO<sub>3</sub>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>] E)[H<sub>2</sub>CO<sub>3</sub>] > [HCO<sub>3</sub><sup>-</sup>] and [HPO<sub>4</sub><sup>2</sup><sup>-</sup>] > [H<sub>2</sub>PO<sub>4</sub><sup>-</sup>]](https://d2lvgg3v3hfg70.cloudfront.net/TB6423/11eaa8f0_66e6_cb5d_93a6_974fa267e285_TB6423_11.jpg) HPO42- + H+ pKa = 7.2
HPO42- + H+ pKa = 7.2At pH 6.4,which one of the following is true of the relative amounts of acid and conjugate base present?
A)[H2CO3] > [HCO3-] and [H2PO4-] > [HPO42-]
B)[H2CO3] = [HCO3-] and [H2PO4-] > [HPO42-]
C)[H2CO3] = [HCO3-] and [HPO42-] > [H2PO4-]
D)[HCO3-] > [H2CO3] and [HPO42-] > [H2PO4-]
E)[H2CO3] > [HCO3-] and [HPO42-] > [H2PO4-]

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
25
You are given 5.00 mL of an H2SO4 solution of unknown concentration.You divide the 5.00-mL sample into five 1.00-mL samples and titrate each separately with 0.1000 M NaOH.In each titration the H2SO4 is completely neutralized.The average volume of NaOH solution used to reach the endpoint is 15.6 mL.What was the concentration of H2SO4 in the 5.00-mL sample?
A)1.56 M
B)3.90 M
C)0.780 M
D)0.156 M
E)7.80 M
A)1.56 M
B)3.90 M
C)0.780 M
D)0.156 M
E)7.80 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
26
Calculate the pH of a solution that is 2.00 M HF,1.00 M NaOH,and 0.642 M NaF.(Ka = 7.2 10-4)
A)3.14
B)3.36
C)2.65
D)2.93
E)none of these
A)3.14
B)3.36
C)2.65
D)2.93
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
27
In titrating 0.20 M hydrochloric acid,HCl,with 0.20 M NaOH at 25°C,the solution at the equivalence point is
A)0.20 M NaCl
B)very acidic
C)slightly acidic
D)0.10 M HCl and 0.20 M NaOH
E)0.10 M NaCl
A)0.20 M NaCl
B)very acidic
C)slightly acidic
D)0.10 M HCl and 0.20 M NaOH
E)0.10 M NaCl

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following solutions will be the best buffer at a pH of 9.26? (Ka for HC2H3O2 is 1.8 10-5,Kb for NH3 is 1.8 10-5).
A)0.10 M HC2H3O2 and 0.10 M Na C2H3O2
B)5.0 M HC2H3O2 and 5.0 M Na C2H3O2
C)0.10 M NH3 and 0.10 M NH4Cl
D)5.0 M NH3 and 5.0 M NH4Cl
E)5.0 M HC2H3O2 and 5.0 M NH3
A)0.10 M HC2H3O2 and 0.10 M Na C2H3O2
B)5.0 M HC2H3O2 and 5.0 M Na C2H3O2
C)0.10 M NH3 and 0.10 M NH4Cl
D)5.0 M NH3 and 5.0 M NH4Cl
E)5.0 M HC2H3O2 and 5.0 M NH3

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
29
A solution contains 0.250 M HA (Ka = 1.0 10-6)and 0.45 M NaA.What is the pH after 0.17 mole of HCl is added to 1.00 L of this solution?
A)0.77
B)7.82
C)5.82
D)1.97
E)8.18
A)0.77
B)7.82
C)5.82
D)1.97
E)8.18

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
30
Given 100.0 mL of a buffer that is 0.50 M in HOCl and 0.77 M in NaOCl,what is the pH after 10.0 mL of 1.0 M NaOH has been added? (Ka for HOCl = 3.5 10-8)
A)7.68
B)7.74
C)7.46
D)7.12
E)7.79
A)7.68
B)7.74
C)7.46
D)7.12
E)7.79

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
31
How many moles of HCl need to be added to 150.0 mL of 0.50 M NaZ to have a solution with a pH of 6.50? (Ka of HZ is 2.3 10-5)? Assume negligible volume of the HCl.
A)6.8 10-3
B)7.5 10-2
C)5.0 10-1
D)1.0 10-3
E)none of these
A)6.8 10-3
B)7.5 10-2
C)5.0 10-1
D)1.0 10-3
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
32
How many moles of solid NaF would have to be added to 1.0 L of 2.39 M HF solution to achieve a buffer of pH 3.35? Assume there is no volume change.(Ka for HF = 7.2 10-4)
A)3.9
B)0.50
C)0.67
D)1.0
E)1.6
A)3.9
B)0.50
C)0.67
D)1.0
E)1.6

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
33
Calculate the pH of a solution that is 0.50 M in HF (Ka = 7.2 10-4)and 0.95 M in NaF.
A)3.14
B)3.42
C)0.28
D)10.58
E)2.86
A)3.14
B)3.42
C)0.28
D)10.58
E)2.86

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
34
The following question refers to the following system: A 1.0-liter solution contains 0.25 M HF and 0.83 M NaF (Ka for HF is 7.2 10-4). What is the pH of this solution?
A)3.14
B)3.66
C)2.62
D)0.52
E)10.34
A)3.14
B)3.66
C)2.62
D)0.52
E)10.34

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
35
Consider a solution of 2.0 M HCN and 1.0 M NaCN (Ka for HCN = 6.2 10-10).Which of the following statements is true?
A)The solution is not a buffer because [HCN] is not equal to [CN-].
B)The pH will be below 7.00 because the concentration of the acid is greater than that of the base.
C)[OH-] > [H+]
D)The buffer will be more resistant to pH changes from addition of strong acid than of strong base.
E)All of the above are false.
A)The solution is not a buffer because [HCN] is not equal to [CN-].
B)The pH will be below 7.00 because the concentration of the acid is greater than that of the base.
C)[OH-] > [H+]
D)The buffer will be more resistant to pH changes from addition of strong acid than of strong base.
E)All of the above are false.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
36
How many mmoles of HCl must be added to 100 mL of a 0.100 M solution of methylamine (pKb = 3.36)to give a buffer having a pH of 10.00?
A)8.1
B)18.7
C)20.0
D)41.5
E)12.7
A)8.1
B)18.7
C)20.0
D)41.5
E)12.7

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
37
Buffered solution 1 has a greater buffering capacity than buffered solution 2.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
38
A solution contains 0.500 M HA (Ka = 1.0 10-8)and 0.432 M NaA.What is the [H+] after 0.10 mole of HCl is added to 1.00 L of this solution?
A)1.0 10-8 M
B)3.0 10-8 M
C)5.5 1021 M
D)1.8 10-8 M
E)none of these
A)1.0 10-8 M
B)3.0 10-8 M
C)5.5 1021 M
D)1.8 10-8 M
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
39
What is the pH of a solution that results when 0.010 mol HNO3 is added to 500.mL of a solution that is 0.10 M in aqueous ammonia and 0.55 M in ammonium nitrate.Assume no volume change.(The Kb for NH3 = 1.8 10-5. )
A)9.26
B)5.05
C)10.11
D)8.40
E)8.71
A)9.26
B)5.05
C)10.11
D)8.40
E)8.71

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
40
One milliliter (1.00 mL)of acid taken from a lead storage battery is pipetted into a flask.Water and phenolphthalein indicator are added,and the solution is titrated with 0.55 M NaOH until a pink color appears;12.0 mL are required.The number of grams of H2SO4 (formula weight = 98)present in one liter of the battery acid is:
A)647
B)323
C)30
D)1294
E)54
A)647
B)323
C)30
D)1294
E)54

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
41
A 50.00-mL sample of 0.100 M KOH is titrated with 0.202 M HNO3.Calculate the pH of the solution after 52.00 mL of HNO3 is added.
A)12.73
B)0.99
C)1.27
D)13.01
E)none of these
A)12.73
B)0.99
C)1.27
D)13.01
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the following information about the diprotic acid,ascorbic acid.(H2As for short,molar mass 176.1)
H2As HAs- + H+ pKa
HAs- + H+ pKa
 = 4.10 (Ka
= 4.10 (Ka
 = 7.9 10-5)
= 7.9 10-5)
HAs- As2- + H+ pKa
As2- + H+ pKa
 = 11.79 (Ka
= 11.79 (Ka
 = 1.6 10-12)
= 1.6 10-12)
The titration curve for disodium ascorbate,Na2As,with standard HCl is shown below: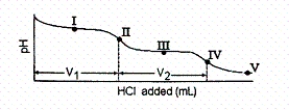
-What major species is (are)present at point III?
A)As2- and HAs-
B)HAs- only
C)HAs- and H2As
D)H2As only
E)H2As and H+
H2As
 HAs- + H+ pKa
HAs- + H+ pKa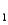 = 4.10 (Ka
= 4.10 (Ka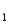 = 7.9 10-5)
= 7.9 10-5)HAs-
 As2- + H+ pKa
As2- + H+ pKa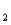 = 11.79 (Ka
= 11.79 (Ka = 1.6 10-12)
= 1.6 10-12)The titration curve for disodium ascorbate,Na2As,with standard HCl is shown below:

-What major species is (are)present at point III?
A)As2- and HAs-
B)HAs- only
C)HAs- and H2As
D)H2As only
E)H2As and H+

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
43
A solution of hydrochloric acid of unknown concentration was titrated with 0.23 M NaOH.If a 100.-mL sample of the HCl solution required exactly 10.mL of the NaOH solution to reach the equivalence point,what was the pH of the HCl solution?
A)12.4
B)1.6
C)-0.4
D)3.3
E)6.6
A)12.4
B)1.6
C)-0.4
D)3.3
E)6.6

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
44
A titration of 200.0 mL of 1.00 M H2A was done with 1.38 M NaOH.For the diprotic acid H2A,Ka1 = 2.5 10-5,Ka2 = 3.1 10-9.Calculate the pH after 100.0 mL of 1.38 M NaOH have been added.
A)9.05
B)8.86
C)5.14
D)9.90
E)4.95
A)9.05
B)8.86
C)5.14
D)9.90
E)4.95

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
45
A titration of 200.0 mL of 1.00 M H2A was done with 1.02 M NaOH.For the diprotic acid H2A,Ka1 = 2.5 10-5,Ka2 = 3.1 10-9.Calculate the pH after 600.0 mL of 1.02 M NaOH have been added.
A)13.423
B)0.577
C)13.712
D)0.288
E)9.423
A)13.423
B)0.577
C)13.712
D)0.288
E)9.423

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
46
A 75.0-mL sample of 0.0500 M HCN (Ka = 6.2 10-10)is titrated with 0.277 M NaOH.What is the [H+] in the solution after 3.0 mL of 0.277 M NaOH have been added?
A)4.6 10-6 M
B)1.0 10-7 M
C)3.5 M
D)2.2 10-9 M
E)none of these
A)4.6 10-6 M
B)1.0 10-7 M
C)3.5 M
D)2.2 10-9 M
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
47
A student titrates an unknown weak acid,HA,to a pale pink phenolphthalein endpoint with 25.0 mL of 0.100 M NaOH.The student then adds 13.0 mL of 0.100 M HCl.The pH of the resulting solution is 4.7.Which of the following is true?
A)At pH 4.7,half the conjugate base,A-,has been converted to HA.
B)The pKa of the acid is 4.7.
C)The pKa of the acid is less than 4.7.
D)The pKa of the acid is greater than 4.7.
E)More than one of the above is correct.
A)At pH 4.7,half the conjugate base,A-,has been converted to HA.
B)The pKa of the acid is 4.7.
C)The pKa of the acid is less than 4.7.
D)The pKa of the acid is greater than 4.7.
E)More than one of the above is correct.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
48
A 50.0-mL sample of 0.10 M HNO2 (Ka = 4.0 10-4)is titrated with 0.12 M NaOH.The pH after 25.0 mL of NaOH have been added is
A)10.43
B)7.00
C)6.57
D)3.57
E)none of these
A)10.43
B)7.00
C)6.57
D)3.57
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
49
A 100.0-mL sample of 0.503 M H2A (diprotic acid)is titrated with 0.200 M NaOH.After 125.0 mL of 0.200 M NaOH has been added,the pH of the solution is 4.50.Calculate Ka1 for H2A.
A)3.2 10-10
B)4.5
C)3.1 10-5
D)not enough information to calculate
E)none of these
A)3.2 10-10
B)4.5
C)3.1 10-5
D)not enough information to calculate
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
50
What quantity of NaOH(s)must be added to 2.00 L of 0.434 M HCl to achieve a pH of 13.00? (Assume no volume change. )
A)0.67 mol
B)1.07 mol
C)0.20 mol
D)1.00 10-13 mol
E)none of these
A)0.67 mol
B)1.07 mol
C)0.20 mol
D)1.00 10-13 mol
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
51
The pH at the equivalence point of the titration of a strong acid with a strong base is:
A)3.9
B)4.5
C)7.0
D)8.2
E)none of these
A)3.9
B)4.5
C)7.0
D)8.2
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
52
Consider the titration of 300.0 mL of 0.450 M NH3 (Kb = 1.8 10-5)with 0.450 M HNO3.How many milliliters of 0.450 M HNO3 are required to reach the stoichiometric point of the reaction?
A)3.50 102 mL
B)4.00 102 mL
C)4.50 102 mL
D)3.00 102 mL
E)none of these
A)3.50 102 mL
B)4.00 102 mL
C)4.50 102 mL
D)3.00 102 mL
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
53
A 19.0-mL sample of tartaric acid is titrated to a phenolphthalein endpoint with 20.mL of 1.0 M NaOH.Assuming tartaric acid is diprotic,what is the molarity of the acid?
A)1.0 M
B)0.53 M
C)2.0 M
D)1.05 M
E)impossible to determine
A)1.0 M
B)0.53 M
C)2.0 M
D)1.05 M
E)impossible to determine

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
54
A titration of 200.0 mL of 1.32 M H2A was done with 1.00 M NaOH.For the diprotic acid H2A,Ka1 = 2.5 10-5,Ka2 = 3.1 10-9.Calculate the pH before any 1.00 M NaOH has been added.
A)11.76
B)4.48
C)9.52
D)8.96
E)2.24
A)11.76
B)4.48
C)9.52
D)8.96
E)2.24

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
55
Consider the titration of 300.0 mL of 0.450 M NH3 (Kb = 1.8 10-5)with 0.450 M HNO3.At the stoichiometric point of this titration,the pH is:
A)4.80
B)2.70
C)4.95
D)4.74
E)7.00
A)4.80
B)2.70
C)4.95
D)4.74
E)7.00

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
56
Consider the titration of 500.0 mL of 0.200 M NaOH with 0.800 M HCl.How many milliliters of 0.800 M HCl must be added to reach a pH of 13.000?
A)55.6 mL
B)24.6 mL
C)18.5 mL
D)12.9 mL
E)4.32 mL
A)55.6 mL
B)24.6 mL
C)18.5 mL
D)12.9 mL
E)4.32 mL

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
57
Consider the titration of 300.0 mL of 0.577 M NH3 (Kb = 1.8 10-5)with 0.500 M HNO3.After 150.0 mL of 0.500 M HNO3 have been added,the pH of the solution is:
A)4.63
B)11.37
C)6.37
D)9.37
E)none of these
A)4.63
B)11.37
C)6.37
D)9.37
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
58
What is the molarity of a sodium hydroxide solution if 28.7 mL of this solution reacts exactly with 22.30 mL of 0.253 M sulfuric acid?
A)0.197 M
B)0.786 M
C)7.26 M
D)0.393 M
E)0.221 M
A)0.197 M
B)0.786 M
C)7.26 M
D)0.393 M
E)0.221 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
59
If 25 mL of 0.750 M HCl are added to 100.mL of 0.352 M NaOH,what is the final pH?
A)13.12
B)0.88
C)13.45
D)0.55
E)7.00
A)13.12
B)0.88
C)13.45
D)0.55
E)7.00

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
60
If 25.0 mL of 0.451 M NaOH solution is titrated with 0.253 M H2SO4,the flask at the endpoint will contain (besides the indicator phenolphthalein)as the principal components:
A)sodium hydroxide,sulfuric acid,and water
B)dissolved sodium sulfate and water
C)sodium hydroxide,sodium sulfate,and water
D)dissolved sodium sulfate,sulfuric acid,and water
E)precipitated sodium sulfate and water
A)sodium hydroxide,sulfuric acid,and water
B)dissolved sodium sulfate and water
C)sodium hydroxide,sodium sulfate,and water
D)dissolved sodium sulfate,sulfuric acid,and water
E)precipitated sodium sulfate and water

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
61
A solution contains 10.mmol of H3PO4 and 5.0 mmol of NaH2PO4.How many milliliters of 0.10 M NaOH must be added to reach the second equivalence point of the titration of the H3PO4 with NaOH?
A)250
B)150
C)1.0 102
D)50
E)2.0 102
A)250
B)150
C)1.0 102
D)50
E)2.0 102

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
62
A solution containing 10.mmol of 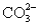 and 5.0 mmol of
and 5.0 mmol of 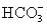 is titrated with 1.7 M HCl.What total volume of HCl must be added to reach the second equivalence point?
is titrated with 1.7 M HCl.What total volume of HCl must be added to reach the second equivalence point?
A)8.8 mL
B)5.9 mL
C)2.9 mL
D)14.7 mL
E)19.7 mL
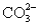 and 5.0 mmol of
and 5.0 mmol of 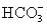 is titrated with 1.7 M HCl.What total volume of HCl must be added to reach the second equivalence point?
is titrated with 1.7 M HCl.What total volume of HCl must be added to reach the second equivalence point?A)8.8 mL
B)5.9 mL
C)2.9 mL
D)14.7 mL
E)19.7 mL

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
63
You have 100.0 mL of 0.100 M aqueous solutions of each of the following acids: HCN,HF,HCl,and HC2H3O2.You titrate each with 0.100 M NaOH (aq).Rank the pHs of each of the solutions when each are titrated to the equivalence point,from highest to lowest pH. Ka for HCN = 6.2 10-10
Ka for HF = 7.2 10-4
Ka for HC2H3O2 = 1.8 10-5
A)HCN,HC2H3O2,HF,HCl
B)HCl,HF,HCN,HC2H3O2
C)HF,HCN,HC2H3O2,HCl
D)HC2H3O2,HCl,HCN,HF
E)none of these
Ka for HF = 7.2 10-4
Ka for HC2H3O2 = 1.8 10-5
A)HCN,HC2H3O2,HF,HCl
B)HCl,HF,HCN,HC2H3O2
C)HF,HCN,HC2H3O2,HCl
D)HC2H3O2,HCl,HCN,HF
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
64
A 50.00-mL solution of 0.0350 M hydrocyanic acid (Ka = 6.2 10-10)is titrated with a 0.0333 M solution of sodium hydroxide as the titrant.What is the pH of the acid solution after 15.00 mL of titrant have been added? (Kw = 1.00 10-14)
A)1.46
B)5.33
C)8.81
D)10.60
E)9.21
A)1.46
B)5.33
C)8.81
D)10.60
E)9.21

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
65
A 100.0-mL sample of 0.2 M (CH3)3N (Kb = 5.33 10-5)is titrated with 0.2 M HCl.What is the pH at the equivalence point?
A)2.6
B)8.6
C)10.7
D)5.4
E)7.0
A)2.6
B)8.6
C)10.7
D)5.4
E)7.0

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
66
How many of the following will raise the pH of a weak acid HA in aqueous solution?
I.addition of water
II.making a buffered solution by adding NaA(s)
III.addition of NaCl(s)
IV.addition of HNO3
V.titrating with KOH
A)1
B)2
C)3
D)4
E)5
I.addition of water
II.making a buffered solution by adding NaA(s)
III.addition of NaCl(s)
IV.addition of HNO3
V.titrating with KOH
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
67
Consider the following information about the diprotic acid,ascorbic acid.(H2As for short,molar mass 176.1)
H2As HAs- + H+ pKa
HAs- + H+ pKa
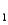 = 4.10 (Ka
= 4.10 (Ka
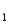 = 7.9 10-5)
= 7.9 10-5)
HAs- As2- + H+ pKa
As2- + H+ pKa
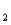 = 11.79 (Ka
= 11.79 (Ka
 = 1.6 10-12)
= 1.6 10-12)
The titration curve for disodium ascorbate,Na2As,with standard HCl is shown below:
-What is the pH at point III?
A)4.10
B)7.95
C)11.79
D)12.39
E)none of these
H2As
 HAs- + H+ pKa
HAs- + H+ pKa = 4.10 (Ka
= 4.10 (Ka = 7.9 10-5)
= 7.9 10-5)HAs-
 As2- + H+ pKa
As2- + H+ pKa = 11.79 (Ka
= 11.79 (Ka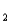 = 1.6 10-12)
= 1.6 10-12)The titration curve for disodium ascorbate,Na2As,with standard HCl is shown below:

-What is the pH at point III?
A)4.10
B)7.95
C)11.79
D)12.39
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
68
Consider the following information about the diprotic acid,ascorbic acid.(H2As for short,molar mass 176.1)
H2As HAs- + H+ pKa
HAs- + H+ pKa
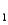 = 4.10 (Ka
= 4.10 (Ka
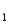 = 7.9 10-5)
= 7.9 10-5)
HAs- As2- + H+ pKa
As2- + H+ pKa
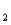 = 11.79 (Ka
= 11.79 (Ka
 = 1.6 10-12)
= 1.6 10-12)
The titration curve for disodium ascorbate,Na2As,with standard HCl is shown below:
-What is the pH at point I (V1/2 HCl added)?
A)4.10
B)7.95
C)11.79
D)12.39
E)none of these
H2As
 HAs- + H+ pKa
HAs- + H+ pKa = 4.10 (Ka
= 4.10 (Ka = 7.9 10-5)
= 7.9 10-5)HAs-
 As2- + H+ pKa
As2- + H+ pKa = 11.79 (Ka
= 11.79 (Ka = 1.6 10-12)
= 1.6 10-12)The titration curve for disodium ascorbate,Na2As,with standard HCl is shown below:

-What is the pH at point I (V1/2 HCl added)?
A)4.10
B)7.95
C)11.79
D)12.39
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
69
Calculate the pH at the equivalence point for the titration of 1.0 M ethylamine,C2H5NH2,by 1.0 M perchloric acid,HClO4.(pKb for C2H5NH2 = 3.25)
A)6.05
B)2.24
C)5.53
D)2.09
E)5.38
A)6.05
B)2.24
C)5.53
D)2.09
E)5.38

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
70
Consider the titration of 100.0 mL of 0.250 M aniline (Kb = 3.8 10-10)with 0.500 M HCl.For calculating the volume of HCl required to reach a pH of 8.0,which of the following expressions is correct? (x = volume in mL of HCl required to reach a pH of 8.0)
A)![<strong>Consider the titration of 100.0 mL of 0.250 M aniline (K<sub>b</sub> = 3.8 \times 10<sup>-</sup><sup>10</sup>)with 0.500 M HCl.For calculating the volume of HCl required to reach a pH of 8.0,which of the following expressions is correct? (x = volume in mL of HCl required to reach a pH of 8.0)</strong> A) = [aniline] B)[H<sup>+</sup>] = x C) = [aniline] D) = [aniline] E)none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6423/11eaa8f0_66e8_7919_93a6_edb0f8d0fe5d_TB6423_11.jpg) = [aniline]
= [aniline]
B)[H+] = x
C)![<strong>Consider the titration of 100.0 mL of 0.250 M aniline (K<sub>b</sub> = 3.8 \times 10<sup>-</sup><sup>10</sup>)with 0.500 M HCl.For calculating the volume of HCl required to reach a pH of 8.0,which of the following expressions is correct? (x = volume in mL of HCl required to reach a pH of 8.0)</strong> A) = [aniline] B)[H<sup>+</sup>] = x C) = [aniline] D) = [aniline] E)none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6423/11eaa8f0_66e8_791a_93a6_a317f0f285a3_TB6423_11.jpg) = [aniline]
= [aniline]
D)![<strong>Consider the titration of 100.0 mL of 0.250 M aniline (K<sub>b</sub> = 3.8 \times 10<sup>-</sup><sup>10</sup>)with 0.500 M HCl.For calculating the volume of HCl required to reach a pH of 8.0,which of the following expressions is correct? (x = volume in mL of HCl required to reach a pH of 8.0)</strong> A) = [aniline] B)[H<sup>+</sup>] = x C) = [aniline] D) = [aniline] E)none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6423/11eaa8f0_66e8_791b_93a6_070ce1b75fa1_TB6423_11.jpg) = [aniline]
= [aniline]
E)none of these
A)
![<strong>Consider the titration of 100.0 mL of 0.250 M aniline (K<sub>b</sub> = 3.8 \times 10<sup>-</sup><sup>10</sup>)with 0.500 M HCl.For calculating the volume of HCl required to reach a pH of 8.0,which of the following expressions is correct? (x = volume in mL of HCl required to reach a pH of 8.0)</strong> A) = [aniline] B)[H<sup>+</sup>] = x C) = [aniline] D) = [aniline] E)none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6423/11eaa8f0_66e8_7919_93a6_edb0f8d0fe5d_TB6423_11.jpg) = [aniline]
= [aniline]B)[H+] = x
C)
![<strong>Consider the titration of 100.0 mL of 0.250 M aniline (K<sub>b</sub> = 3.8 \times 10<sup>-</sup><sup>10</sup>)with 0.500 M HCl.For calculating the volume of HCl required to reach a pH of 8.0,which of the following expressions is correct? (x = volume in mL of HCl required to reach a pH of 8.0)</strong> A) = [aniline] B)[H<sup>+</sup>] = x C) = [aniline] D) = [aniline] E)none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6423/11eaa8f0_66e8_791a_93a6_a317f0f285a3_TB6423_11.jpg) = [aniline]
= [aniline]D)
![<strong>Consider the titration of 100.0 mL of 0.250 M aniline (K<sub>b</sub> = 3.8 \times 10<sup>-</sup><sup>10</sup>)with 0.500 M HCl.For calculating the volume of HCl required to reach a pH of 8.0,which of the following expressions is correct? (x = volume in mL of HCl required to reach a pH of 8.0)</strong> A) = [aniline] B)[H<sup>+</sup>] = x C) = [aniline] D) = [aniline] E)none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6423/11eaa8f0_66e8_791b_93a6_070ce1b75fa1_TB6423_11.jpg) = [aniline]
= [aniline]E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
71
What volume of 0.0100 M NaOH must be added to 1.00 L of 0.0500 M HOCl to achieve a pH of 8.00? The Ka for HOCl is 3.5 10-8.
A)1.0 L
B)5.0 L
C)1.2 L
D)3.9 L
E)none of these
A)1.0 L
B)5.0 L
C)1.2 L
D)3.9 L
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
72
You dissolve 1.15 grams of an unknown diprotic acid in 200.0 mL of H2O.This solution is just neutralized by 5.00 mL of a 1.00 M NaOH solution.What is the molar mass of the unknown acid?
A)230
B)115
C)28.8
D)460
E)none of these
A)230
B)115
C)28.8
D)460
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following is a major species present at point IV?
A)H2As
B)HAs-
C)As2-
D)H+
E)none of these
A)H2As
B)HAs-
C)As2-
D)H+
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
74
A 2.36-g sample of an acid,H2X,requires 45.0 mL of a 0.500 M NaOH solution for complete reaction (removing both protons).The molar mass of the acid is:
A)105
B)255
C)232
D)210
E)none of these
A)105
B)255
C)232
D)210
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
75
A 25.00-mL sample of propanoic acid,CH3CH2COOH,of unknown concentration was titrated with 0.111 M KOH.The equivalence point was reached when 44.54 mL of base had been added.What is the concentration of the propanoate ion at the equivalence point?
A)0.111 M
B)0.0711 M
C)0.198 M
D)0.128 M
E)0.147 M
A)0.111 M
B)0.0711 M
C)0.198 M
D)0.128 M
E)0.147 M

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following is the net ionic equation for the reaction that occurs during the titration of nitric acid with potassium hydroxide?
A)HNO3 + K+ OH- KNO3 + H2O
B)HNO3 + H2O NO3- + H3O+
C)HNO3 + KOH K+ + NO3- + H2O
D)HNO3 + OH- NO3- + H2O
E)H+ + OH- H2O
A)HNO3 + K+ OH- KNO3 + H2O
B)HNO3 + H2O NO3- + H3O+
C)HNO3 + KOH K+ + NO3- + H2O
D)HNO3 + OH- NO3- + H2O
E)H+ + OH- H2O

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
77
Consider the titration of 100.0 mL of 0.250 M aniline (Kb = 3.82 10-10)with 0.500 M HCl.Calculate the pH of the solution at the stoichiometric point.
A)5.10
B)9.42
C)2.68
D)11.32
E)none of these
A)5.10
B)9.42
C)2.68
D)11.32
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
78
A solution containing 10.mmol of 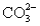 and 5.0 mmol of
and 5.0 mmol of 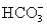 is titrated with 1.8 M HCl.What volume of HCl must be added to reach the first equivalence point?
is titrated with 1.8 M HCl.What volume of HCl must be added to reach the first equivalence point?
A)2.8 mL
B)5.6 mL
C)10.6 mL
D)15.6 mL
E)20.6 mL
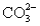 and 5.0 mmol of
and 5.0 mmol of 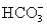 is titrated with 1.8 M HCl.What volume of HCl must be added to reach the first equivalence point?
is titrated with 1.8 M HCl.What volume of HCl must be added to reach the first equivalence point?A)2.8 mL
B)5.6 mL
C)10.6 mL
D)15.6 mL
E)20.6 mL

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
79
A 0.210-g sample of an acid (molar mass = 192 g/mol)is titrated with [$X] mL of [$Y] M NaOH to a phenolphthalein endpoint.The formula of the acid is:
A)HA
B)H2A
C)H3A
D)H4A
E)not enough information given
A)HA
B)H2A
C)H3A
D)H4A
E)not enough information given

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
80
In titrating 100 mL of 0.10 M HCl (aq)with 0.10 M NaOH,the pH does not change a great deal initially.Why is this?
A)The amount of OH- has no affect on the pH of aqueous solutions.
B)The H+ from the HCl (aq)acts like a buffer.
C)The major species when the acid and base are mixed form a buffered solution.
D)The statement "In titrating 100 mL of 0.10 M HCl (aq)with 0.10 M NaOH,the pH does not change a great deal initially." is not true.
E)None of these.
A)The amount of OH- has no affect on the pH of aqueous solutions.
B)The H+ from the HCl (aq)acts like a buffer.
C)The major species when the acid and base are mixed form a buffered solution.
D)The statement "In titrating 100 mL of 0.10 M HCl (aq)with 0.10 M NaOH,the pH does not change a great deal initially." is not true.
E)None of these.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck



